What is Schiemann reaction?
The Schiemann reaction, commonly referred to as the Balz-Schiemann reaction or Balz-Schiemann decomposition, is a widely used method discovered by Balz and Schiemann in 1927 for introducing a fluorine atom into aromatic rings.
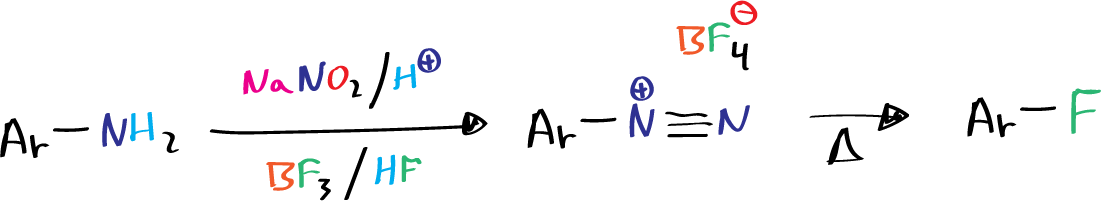
Schiemann reaction involves the conversion of aromatic amines to diazonium fluoroborates, which are then thermally decomposed to produce aryl fluorides. The reaction is particularly significant for synthesizing specifically fluorinated aromatic compounds, with a relatively high yield of aromatic fluorides obtained from the decomposition in the absence of solvent.
Additionally, the general procedure can be directly applied to producing other aromatic halides.
References
- Balz, G. and Schiemann, G. (1927), Über aromatische Fluorverbindungen, I.: Ein neues Verfahren zu ihrer Darstellung. [On aromatic fluorine compounds, I.: A new method for their preparation.] Ber. dtsch. Chem. Ges. A/B, 60: 1186-1190. https://doi.org/10.1002/cber.19270600539
- G. Balz; G. Schiemann, “Process for preparing organic fluorine compounds” .U.S. Patent 1916327 (1933)
- Some Fluorinated Chlorobenzenes1
Harold Simmons Booth, Howard M. Elsey, and Paul E. Burchfield
Journal of the American Chemical Society 1935 57 (11), 2064-2065
DOI: 10.1021/ja01314a012
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.