What is Suzuki cross coupling?
Suzuki cross coupling, also known as Suzuki-Miyaura coupling, is a chemical reaction used to synthesize organic compounds by coupling aryl or vinyl halides with organoboron compounds. The reaction was first reported by Akira Suzuki and Koichi Miyaura in 1979, and it has since become a widely used method in organic chemistry due to its high efficiency and wide substrate scope..
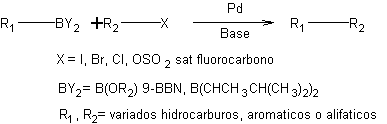
The reaction mechanism of Suzuki coupling involves the formation of a complex between the organoboron compound and a palladium catalyst, which then undergoes oxidative addition to form a palladium-carbon bond. The aryl or vinyl halide is then introduced and undergoes a reductive elimination reaction to form the desired coupling product..
One of the main advantages of Suzuki coupling is its compatibility with a wide range of functional groups, including alkyl, aryl, and vinyl groups. This makes it a useful method for synthesizing a wide range of organic compounds, including pharmaceuticals, agrochemicals, and materials..
Suzuki coupling is also highly efficient, with high yield and good selectivity. It is typically performed under mild reaction conditions, which makes it suitable for synthesizing sensitive and labile compounds..
There are several variations of Suzuki coupling that have been developed to improve its efficiency and scope, including the use of different palladium catalysts and the use of modified reaction conditions. For example, the use of palladium on carbon (Pd/C) as a catalyst has been shown to improve the yield and selectivity of Suzuki coupling..
Summary
Suzuki coupling is a powerful tool for synthesizing a wide range of organic compounds and has become an essential method in organic chemistry..