What is Williamson ether synthesis?
The nucleophilic substitution of an alkali alkoxide on an alkylating reagent to form an ether and an inorganic salt was first reported by Williamson in 1851. This reaction is commonly known as the Williamson ether synthesis, Williamson synthesis or Williamson reaction.
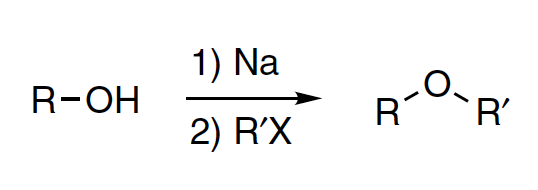
- R = alkyl, aryl
- R’ = primary alkyl, few secondary alkyl
- X = Cl, Br, I, OTs, OMs, OSO2R’, OTf, etc. (see list of acronyms)
Williamson ether synthesis involves two steps:
- generation of alkali alkoxide by the treatment of alcohol or phenol with a strong base
- followed by the nucleophilic substitution of the resulting alkoxide on an alkylating reagent
Symmetrical and asymmetrical ethers can be prepared by this reaction using different alcohols and alkylating reagents. Although the most common alkylating reagents are alkyl halides, dialkyl sulfates, alkyl mesylates, and tosylates are also commonly used. Even nontraditional alkylating reagents such as esters of weak acids, dialkyl ethers, alkyl aryl ethers, and epoxides can be used under special circumstances.
The reaction rate of the Williamson ether synthesis is affected by the solvent, temperature, leaving group on the alkylating reagent, the nucleophilicity of alkoxide, and the counter cation of alkoxide. The amount of free alkoxide dissociates from the alkali alkoxide depends on the size of alkali cation and the polarity of the solvent. The use of crown ethers or iodide salt can accelerate the reaction by complexing with the alkali cation and leaving a free alkoxide anion. A good leaving group on the alkylating reagent is favored, with groups for which the conjugate acids are strong acids considered as good leaving groups, such as iodo and mesylate. Methanol-derived alkoxide is reported to be the most reactive due to its small size. This reaction is often accompanied by elimination as a side reaction if a secondary or tertiary alkylating reagent is used, in which the steric hindrance blocks the backside attack from the alkoxide.
The Williamson ether synthesis is favored by high temperature, especially under extremely high temperature. Under these conditions, esters of weak acids (e.g., acetates and benzoates) and even ethers can be used as the alkylating reagents, as demonstrated by the preparation of anisole from sodium or potassium phenoxide and methanol in the presence of carbon monoxide at 180 ºC.
References
- Williamson, A. (1851), Ueber die Theorie der Aetherbildung. Justus Liebigs Ann. Chem., 77: 37-49. https://doi.org/10.1002/jlac.18510770104
- A.W. Williamson, “XXII.—On etherification” Q. J. Chem. Soc., 1852,4, 229-239
DOI: 10.1039/QJ8520400229