What is Wittig reaction?
The Wittig reaction is a chemical reaction that involves the conversion of an aldehyde or ketone into an alkenyl compound through the use of a phosphonium ylide. This reaction is named after the German chemist Georg Wittig, who first described the reaction in 1954..
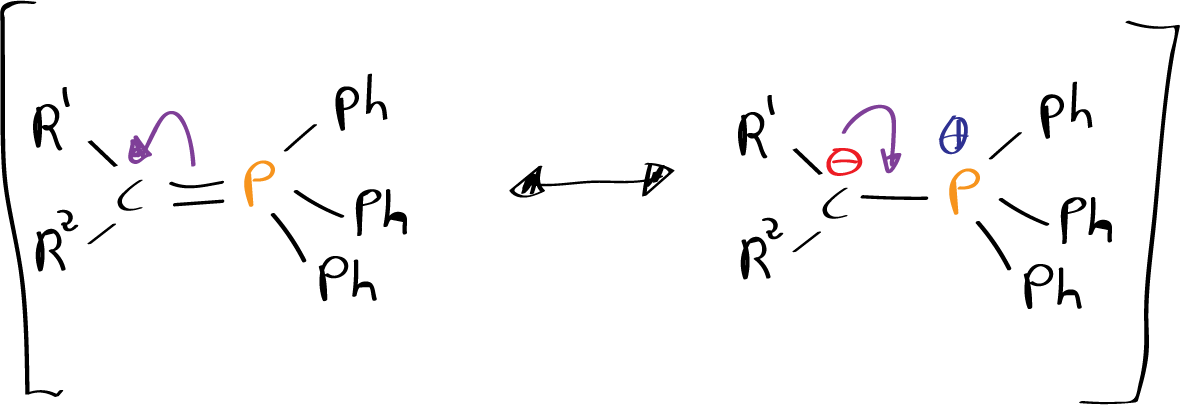
The Wittig reaction is typically performed by adding a phosphonium ylide, also known as a Wittig reagent, to an aldehyde or ketone compound in the presence of a base, such as sodium hydroxide or potassium hydroxide. The phosphonium ylide acts as a nucleophile, attacking the electrophilic carbon atom of the aldehyde or ketone and forming a carbanion..
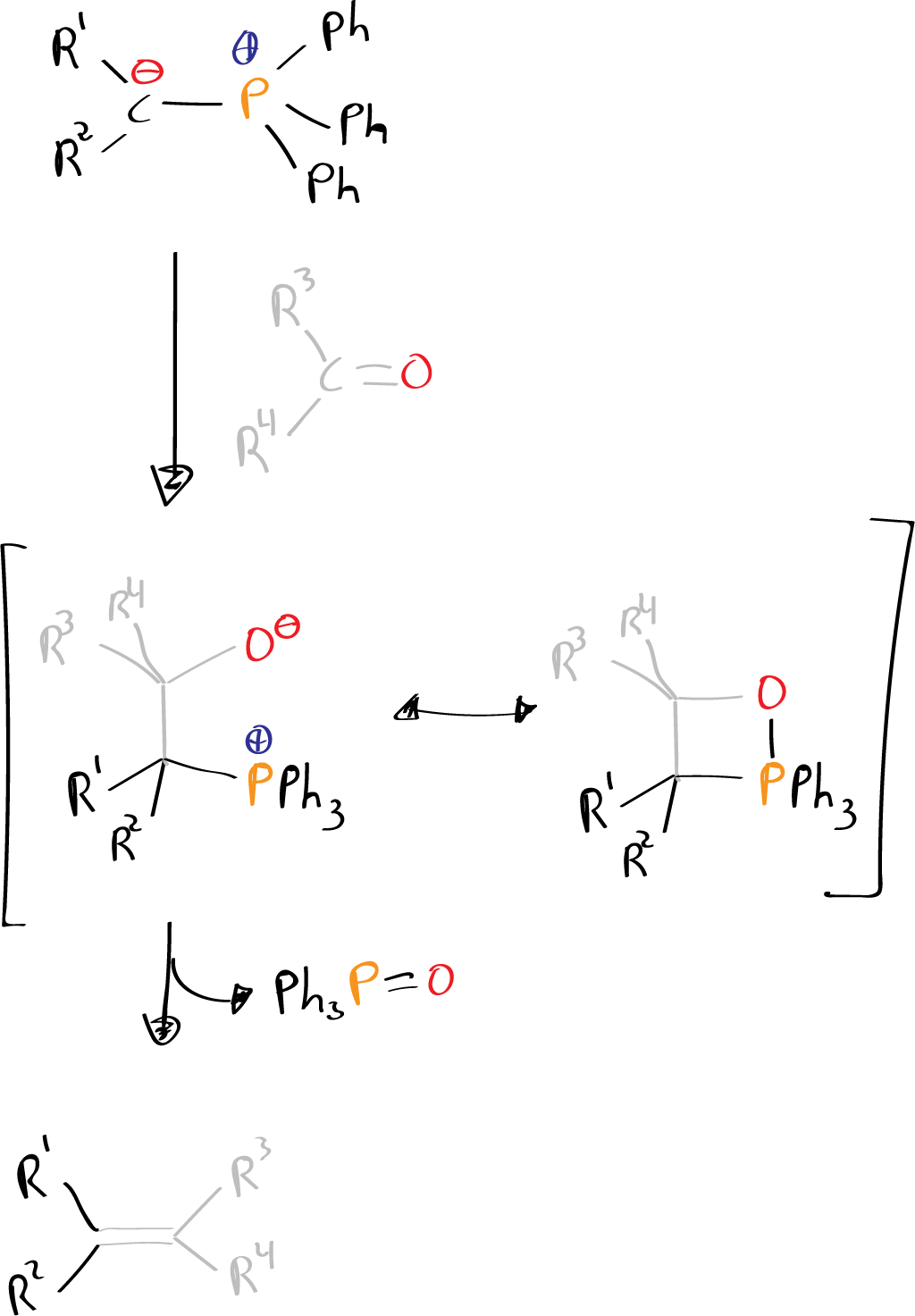
The carbanion is then eliminated through the loss of a proton, resulting in the formation of an alkenyl compound. The Wittig reaction is typically used to synthesize alkenes, but it can also be used to synthesize alkyl compounds under certain conditions..
The Wittig reaction is a useful method for the synthesis of alkenes and alkyl compounds, but it has several limitations, including the need for specialized Wittig reagents and the formation of unwanted byproducts. As a result, it has largely been superseded by other methods, such as the halogenation of alkenes and the hydroboration of alkenes..
Summary
The Wittig reaction is a valuable method for the synthesis of alkenes and alkyl compounds, but it has largely been replaced by more modern and efficient methods. Despite this, it remains an important part of the history of chemistry and continues to be studied by chemists today..
Example
One example of a Wittig reaction is the reaction of triphenylphosphonium ylide with benzaldehyde to form styrene and triphenylphosphonium chloride as a byproduct..
The balanced equation for this reaction is:
Ph3P=CH2 + benzaldehyde → PhCH=CH2 + Ph3PCl
Mechanism of reaction
The mechanism of the Wittig reaction is a multi-step process that involves the formation of an intermediate, known as the Wittig intermediate, before the formation of the alkene product. The basic steps of the mechanism are as follows:
- Protonation of the ylide phosphorus atom by a strong acid such as HCl or HBr, to give an alkylidene-phosphonium ion intermediate.
- Nucleophilic attack of the ylide carbon atom on the electrophilic carbonyl carbon of the aldehyde or ketone, forming a tetrahedral intermediate.
- Deprotonation of the intermediate by a base such as sodium hydroxide, to form an alkene and a phosphonate anion.
- Protonation of the phosphonate anion by the acid used in step 1 to give the final product.
The balanced equation for the whole reaction is:
Ph3P=CH2 + benzaldehyde +H+ (catalyst) → PhCH=CH2 + Ph3P+Cl-
The mechanism can vary depending on the specific reagents and conditions used in the reaction.
References
- G. Wittig, G. and Schöllkopf, U. (1954), Über Triphenyl-phosphin-methylene als olefinbildende Reagenzien (I. Mitteil.) [On triphenyl-phosphine-methylenes as olefin-forming reagents (I. commun.)] Chem. Ber., 87: 1318-1330. https://doi.org/10.1002/cber.19540870919
- Wittig, G. and Haag, W. (1955), Über Triphenyl-phosphinmethylene als olefinbildende Reagenzien (II. Mitteil.1)). [On triphenyl-phosphinemethylenes as olefin-forming reagents (II. commun.1)).] Chem. Ber., 88: 1654-1666. https://doi.org/10.1002/cber.19550881110