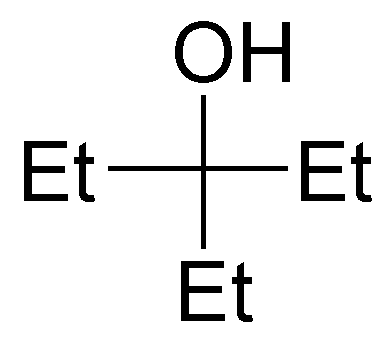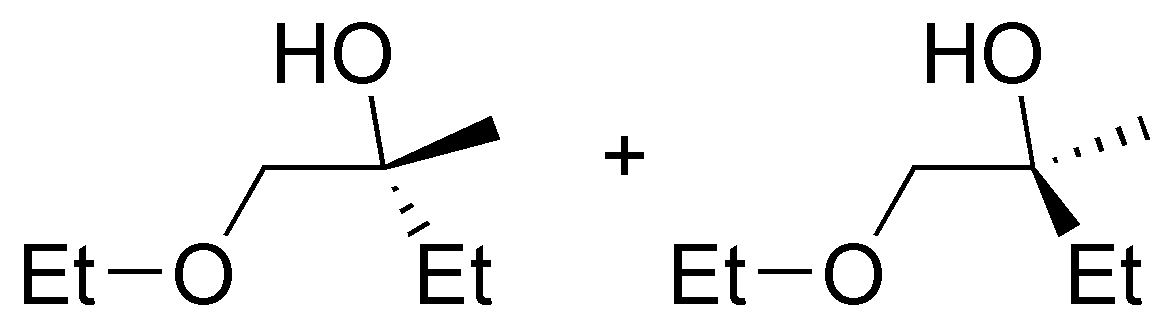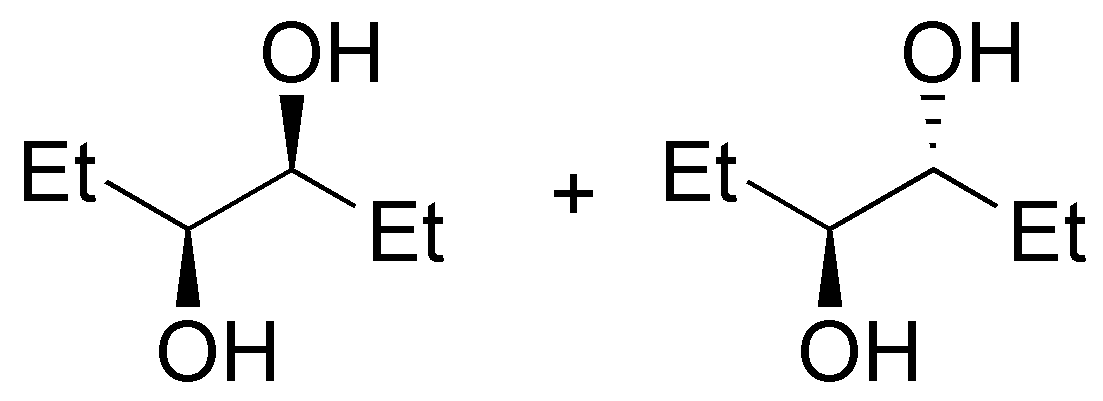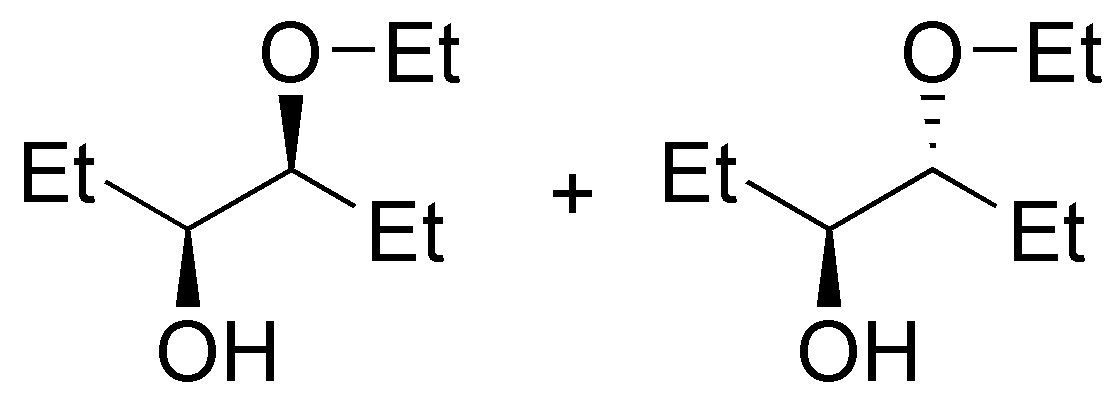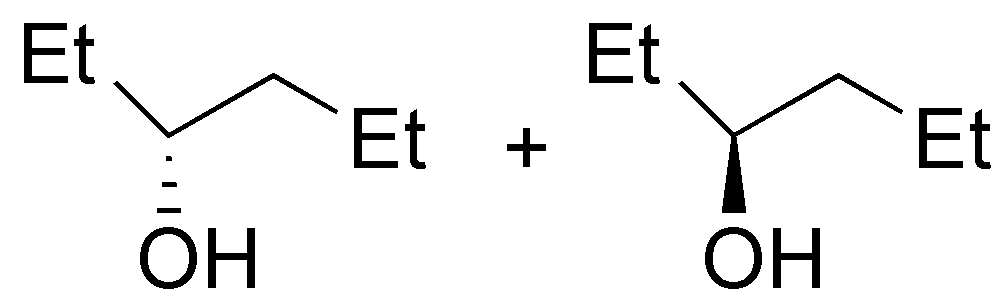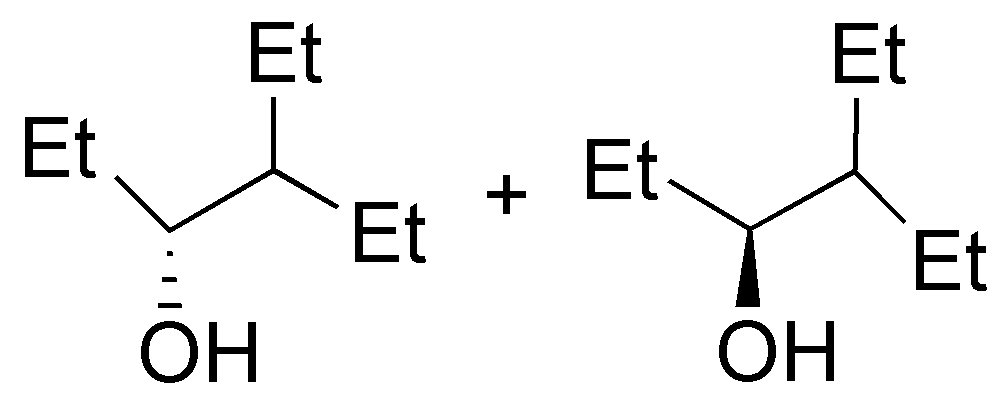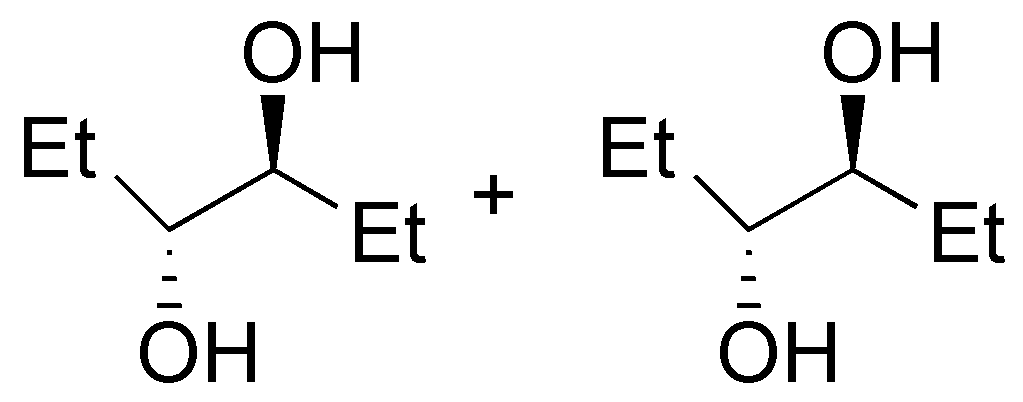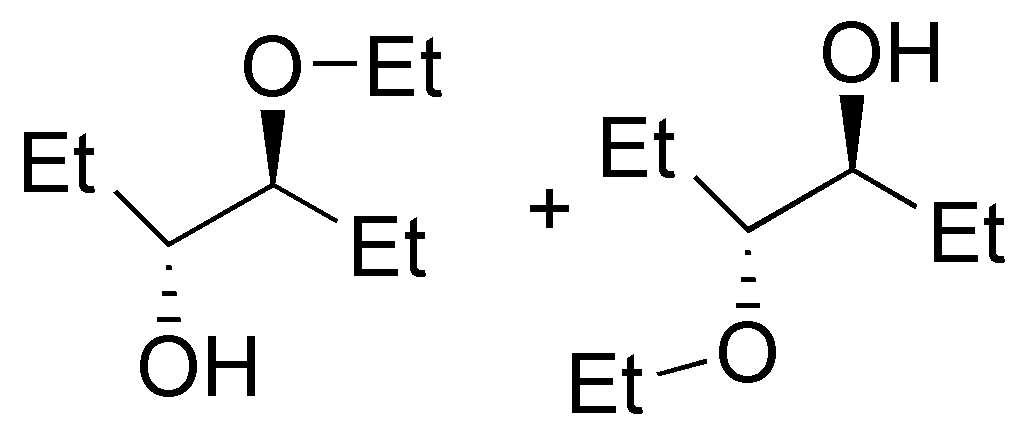Written by J.A Dobado | Last Updated on April 22, 2024
Go to the page with the list of problems.
Alcohols, Ethers and Oxiranes – solutions to the problems
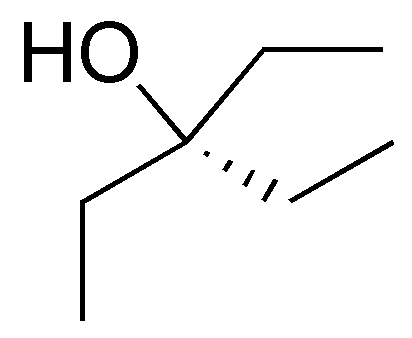
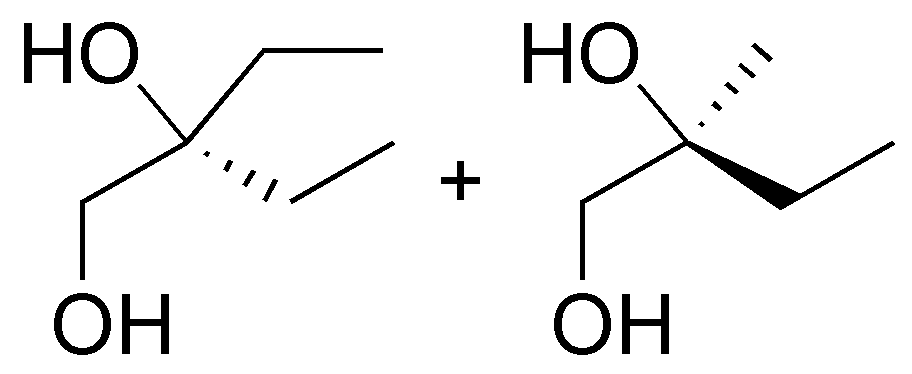
Solution 1:
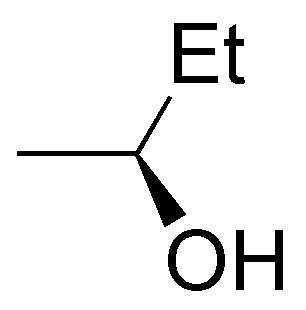
The following alcohols will be primary:
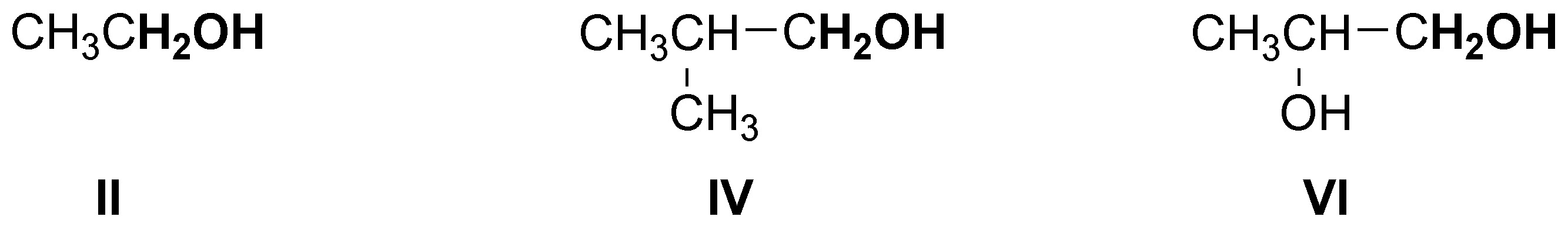
secondary alcohols are bonded to a -CH- as indicated:
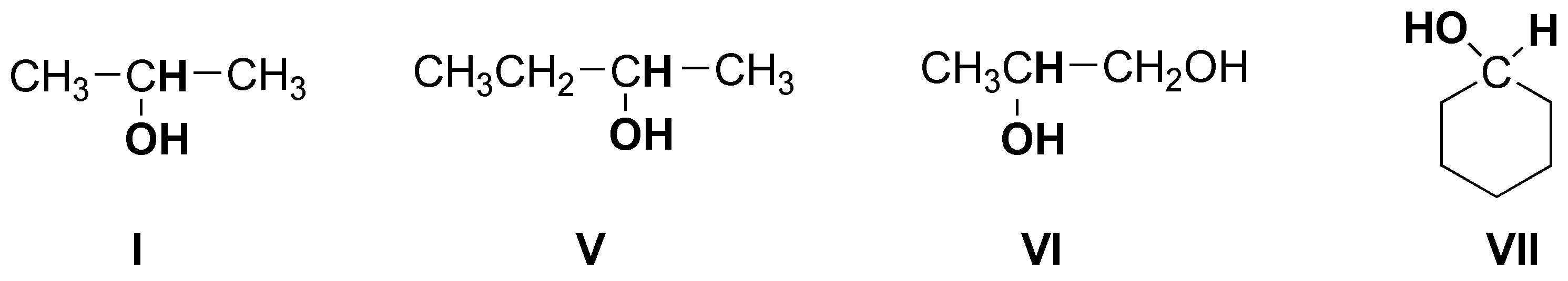
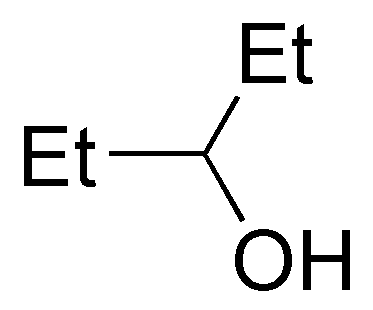
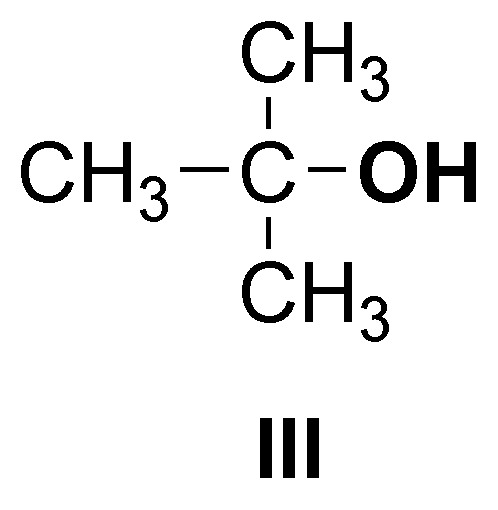
and only compound III is a tertiary alcohol.
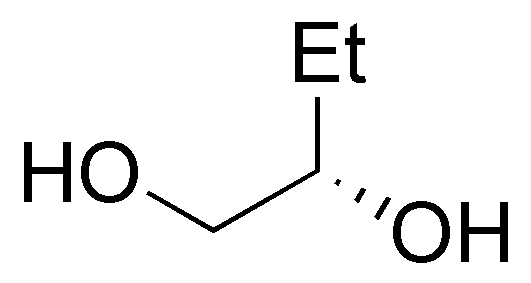
Note that compound VI is a diol (it has two hydroxyls) and can be classified as primary and secondary.
Solution 2:
Alcohols are slightly acidic compounds with pKa values usually ranging from about 15 to 18. The lower the pKa, the more acidic a compound is. The pKa value for alcohols follows the following sequence: 3° > 2° > 1° > MeOH, as a consequence of the positive inductive effect of the alkyl groups (they give up electrons destabilizing the anion). The bases capable of forming the corresponding alkoxides are: metal hydrides, alkali metals or sodium amidide. As for the HO– ion, it is not strong enough to form the alkoxide, however, for the more acidic alcohols (methanol and ethanol) an equilibrium is established so that although RO– is not obtained in stoichiometric quantity, it is present in sufficient proportion to initiate some process, acting as a base. Therefore, the following reagents will form alkoxides with both alcohols: Na, NaNH2 and NaH.
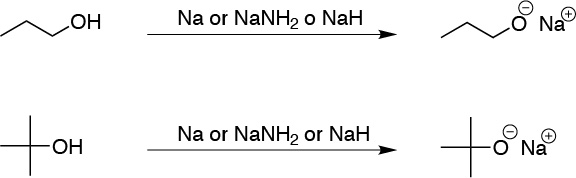
Solution 3:
The first stage of the reaction is the attack of the HBr proton on the hydroxyl group converting it into an alkyl hydronium ion, which readily loses water producing a relatively stable tertiary carbocation which reacts with a bromide ion giving 2-bromo-2-methylbutane as the sole product. It is therefore an SN1.
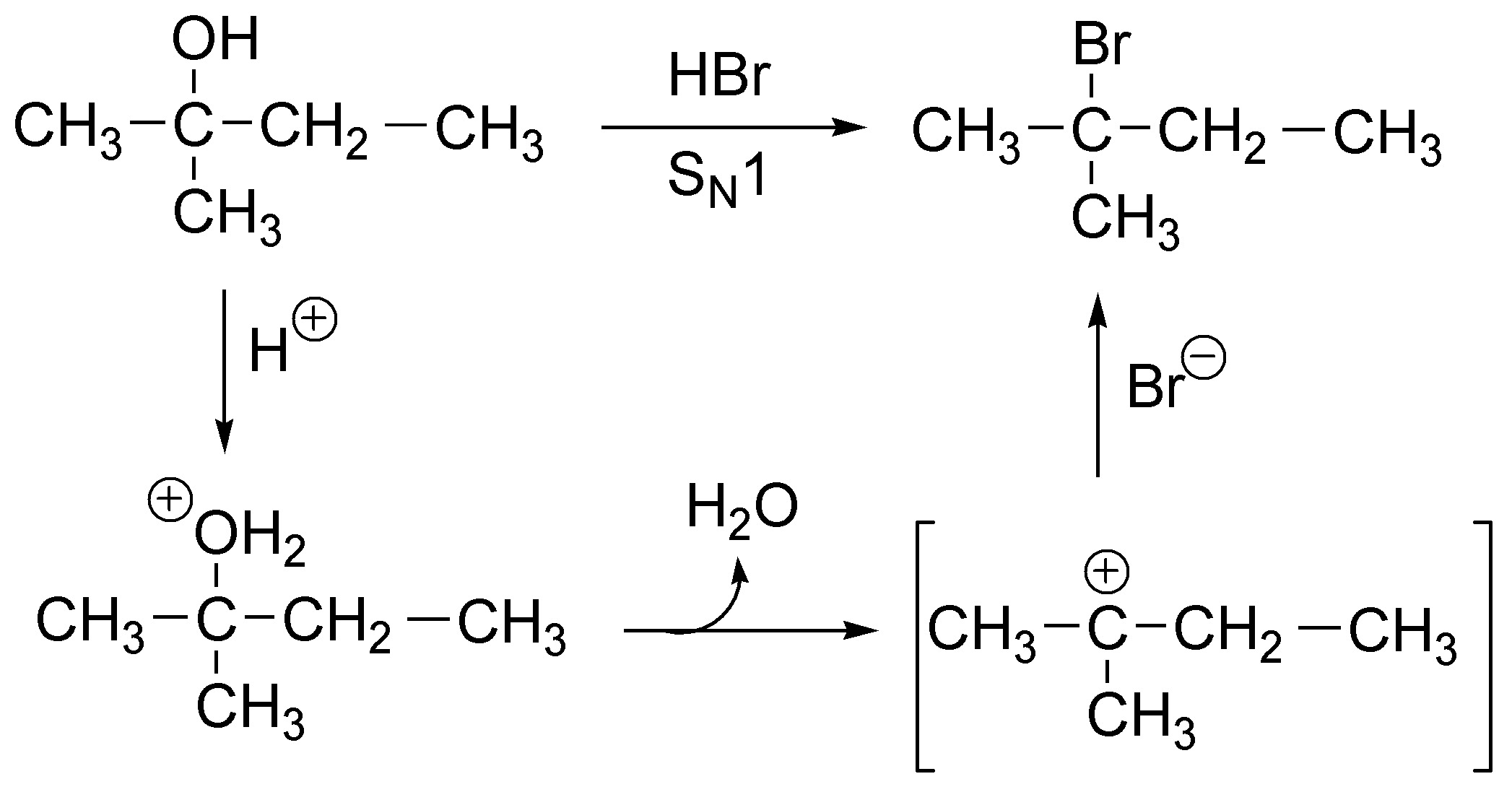
Solution 4:
When dealing with a primary alcohol the protonation produces the alkyl hydronium ion but this does not readily lose water because the carbocation that would be generated would be primary (not very stable) yet it is a good leaving group and facilitates its substitution by a bromide ion via an SN2.
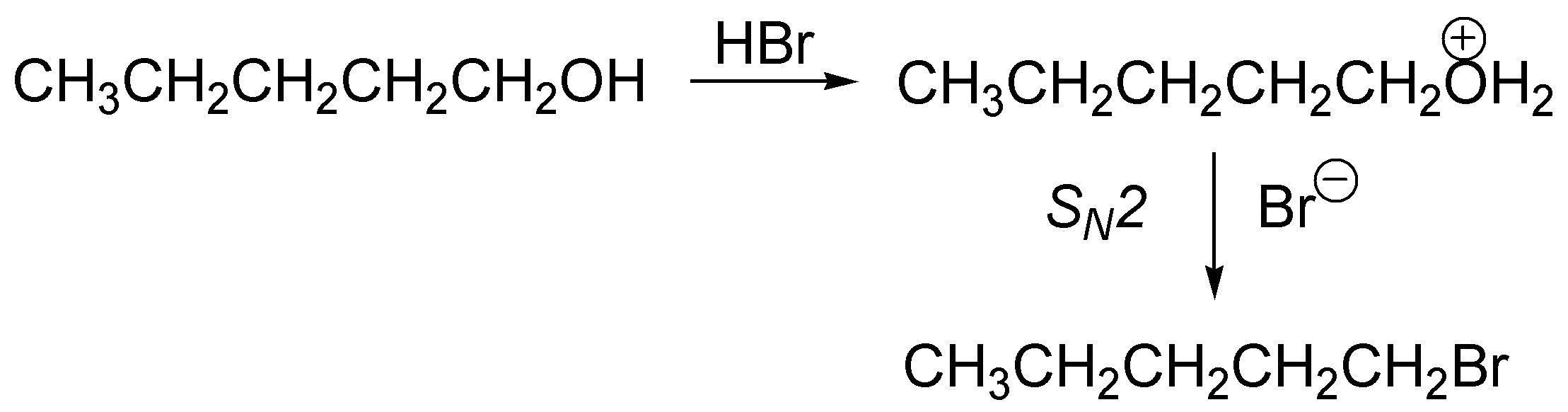
Solution 5:
The solution of ZnCl2 in concentrated HCl is known as Lucas’ reagent and is used for the classification of alcohols. The function of zinc chloride is to convert the hydroxyl group into a better leaving group by forming a complex between the oxygen of the hydroxyl group and zinc favoring the formation of the secondary carbocation which is subsequently attacked by a chloride ion.
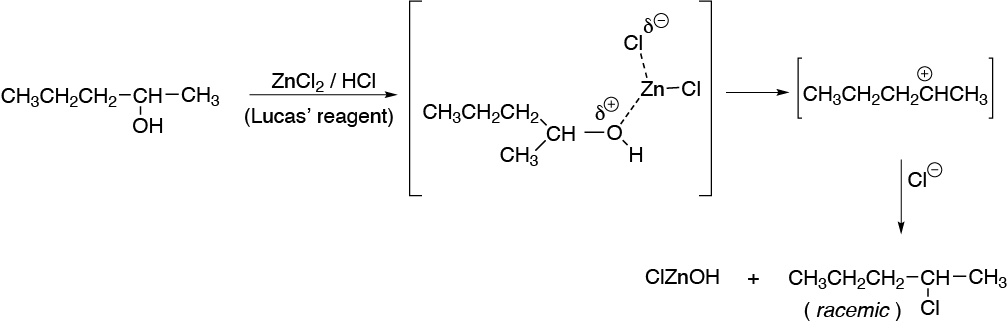
Solution 6:
As it is a primary alcohol the protonation of it produces the alkyl hydronium ion this loses water generating a primary carbocation (not very stable), which in order to stabilize itself evolves to a secondary carbocation by migration (transposition) of a hydrogen, this carbocation (as there is no good nucleophile in the medium) loses a proton producing the most stable alkene (the most substituted), i.e. 2-butene.
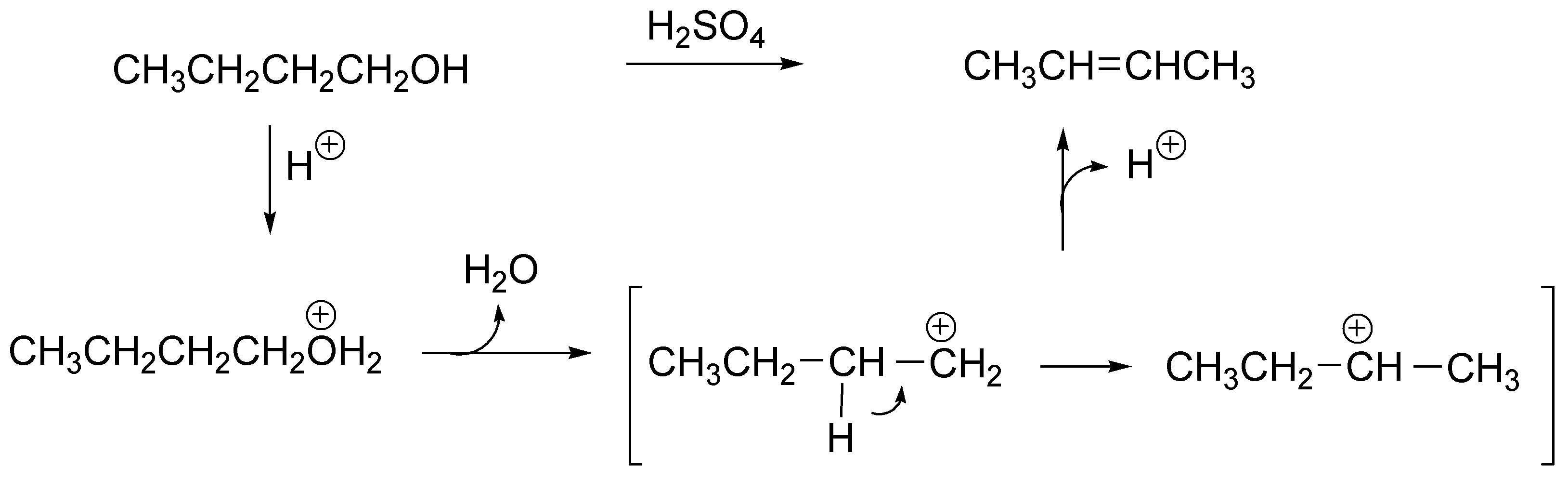
Solution 7:
(a) Butan-1-ol is a primary alcohol. The mineral acid produces the protonation of the -OH group. This is followed by the loss of a water molecule and the formation of a primary carbocation. Since it is very unstable, it evolves into a secondary carbocation, by transposition of a hydrogen, taking a pair of electrons with it, i.e. as a hydride, to give a secondary carbocation. This carbocation evolves into an alkene. The reaction follows Zaitsev’s rule, which indicates that the thermodynamically more stable alkene, which is the most substituted and of trans stereochemistry, is formed in greater proportion.
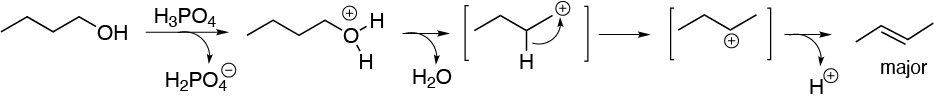
b) 2-methylcyclohexanol generates a secondary carbocation. Of the two possible alkenes, 3-methylcyclohexene and 1-methylcyclohexene, the latter is formed, since it is the more stable compound because it is the more substituted alkene.
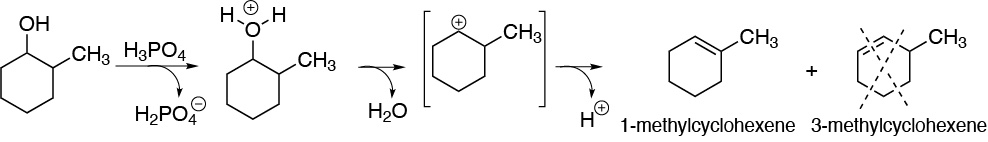
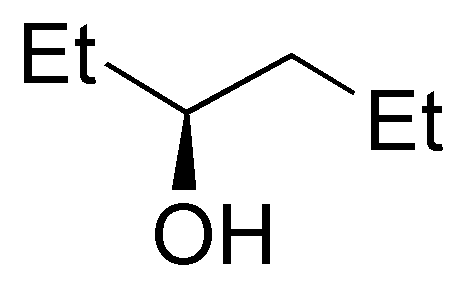
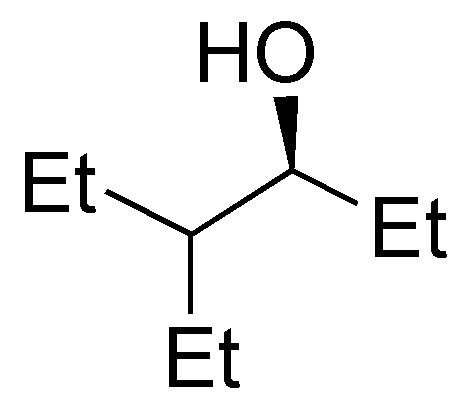
c) 2-methylpentan-3-ol is a secondary alcohol. The carbocation produced by protonation of the hydroxyl group and subsequent exit of a water molecule is also secondary. The loss of hydrogen from carbon 2 leads to the formation of a trisubstituted alkene.
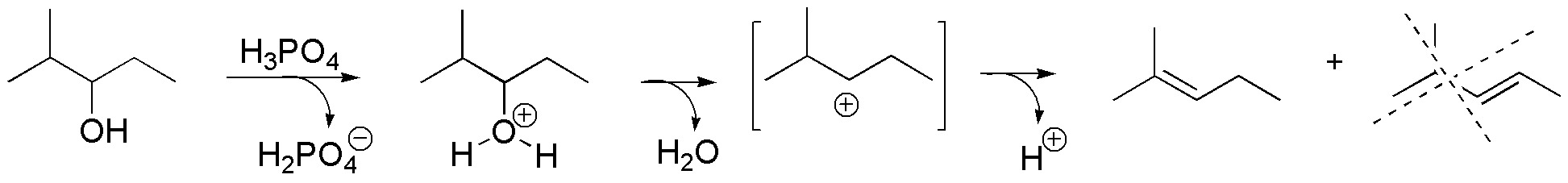
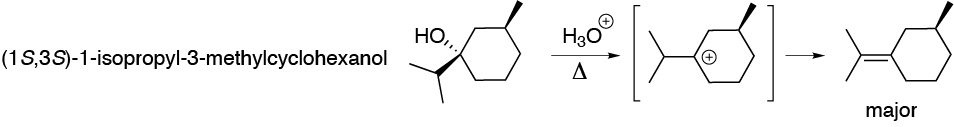
d) Treatment of this chiral alcohol with a mineral acid leads to the formation of a tertiary carbocation. Note that the chirality of the carbon to which the -OH group was attached disappears, while the stereochemistry of carbon 3 is not altered. The reaction ends with the loss of a proton to generate a double bond. The major product comes from the loss of the proton from the isopropyl group, as it gives rise to the most substituted alkene.
Solution 8:
(a) Alkene I could be obtained from alcohols VIII (1-cyclohexyl-1-ethanol) or IX (2-cyclohexyl-1-ethanol), although in the case of VIII a transposition to a tertiary carbocation could occur and give mixed alkenes.
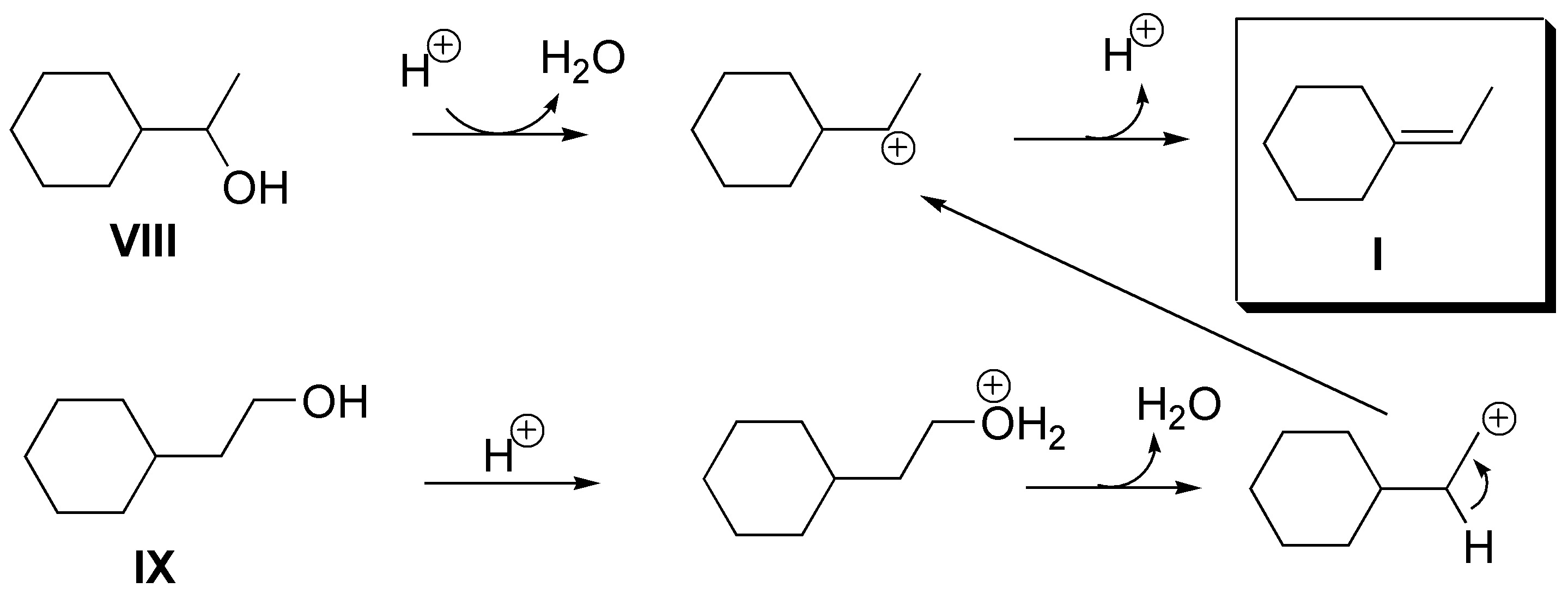
b) The best candidate is 3-methylcyclohexanol (X) although dehydration can produce 4-methylcyclohexene (VII) together with the desired alkene II.
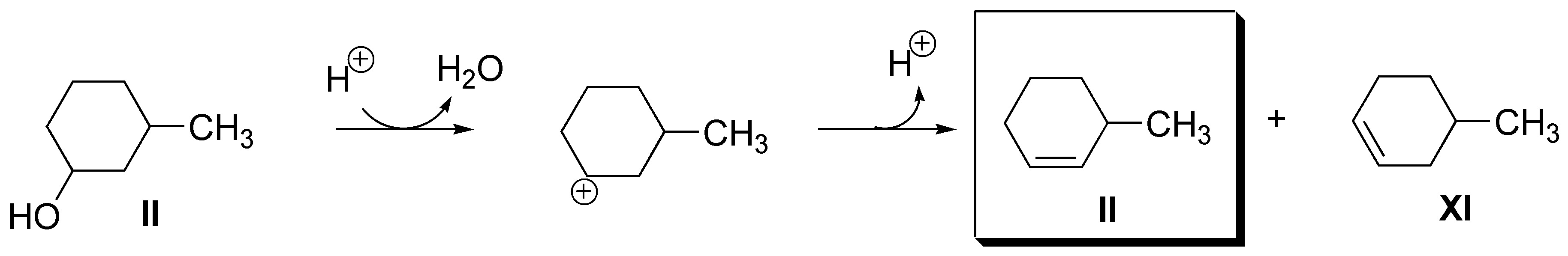
c) There are two possible alcohols: 2-methylcyclohexanol (XI) or 1-methylcyclohexanol (XII):
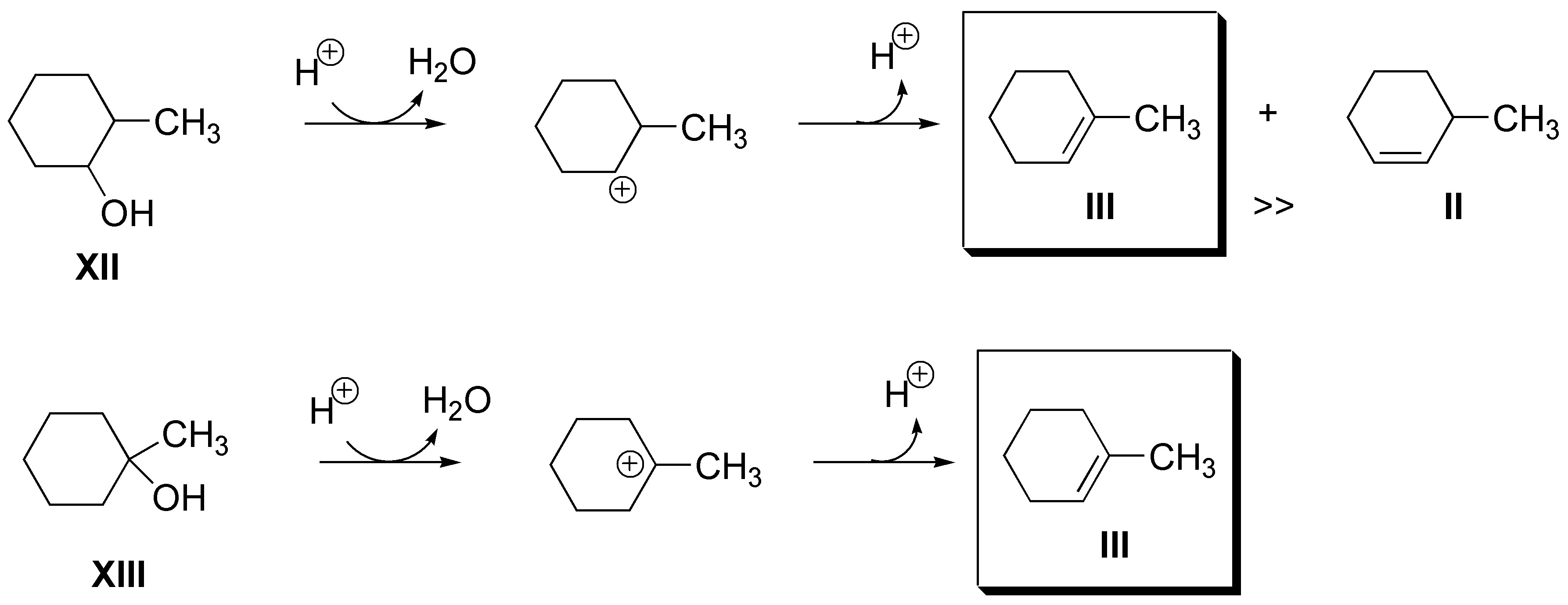
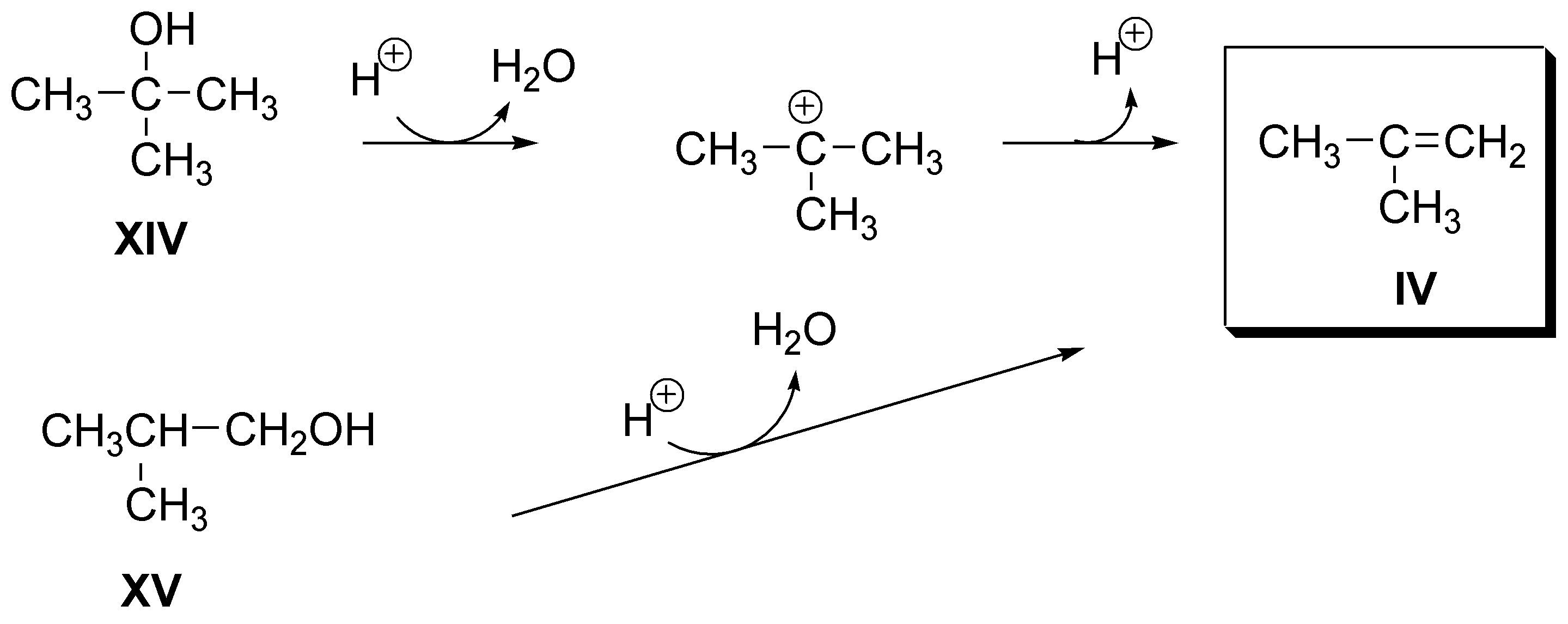
d) Also for alkene IV (2-methylpropene) there will be two possibilities: 2-methyl-2-propanol (XIII) and 2-methyl-1-propanol (XIV), both produce it as the only product:
e) Alkene V (styrene) can be obtained as a single dehydration product from both 1-phenyl-1-ethanol (XV) and 2-phenyl-1-ethanol (XVI):
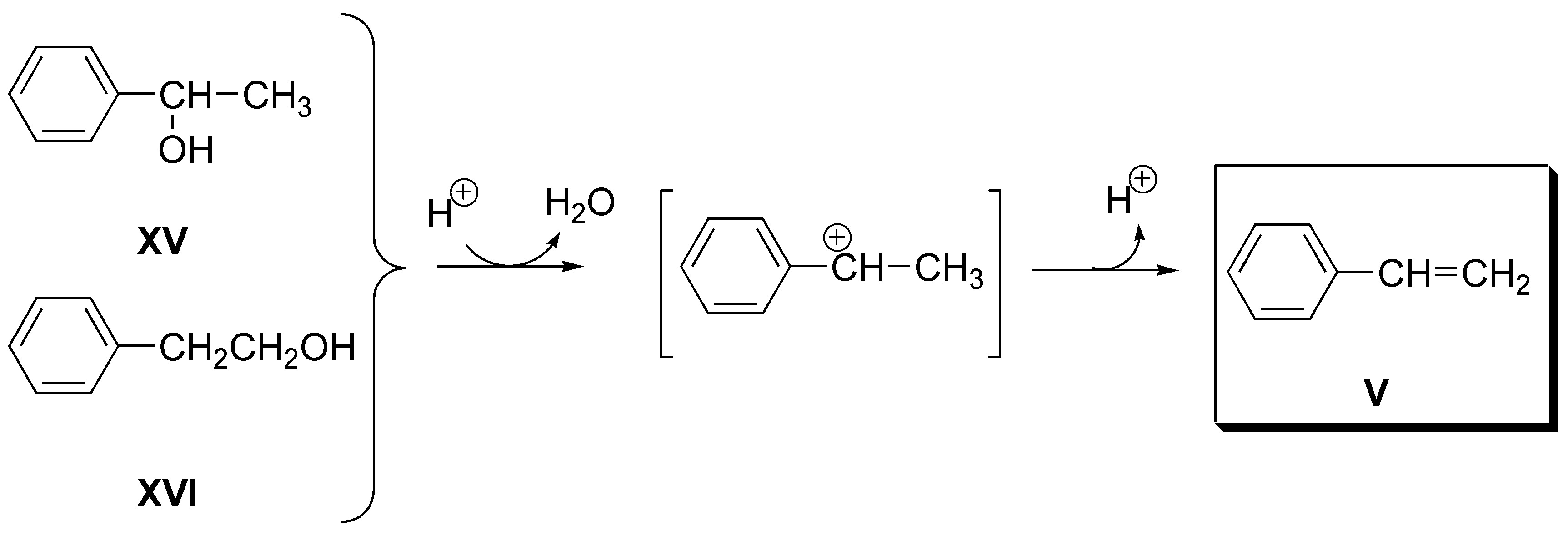
f) 2-pentene (VI) can be obtained from both 2-pentanol (XVII), and 3-pentanol (XVIII):
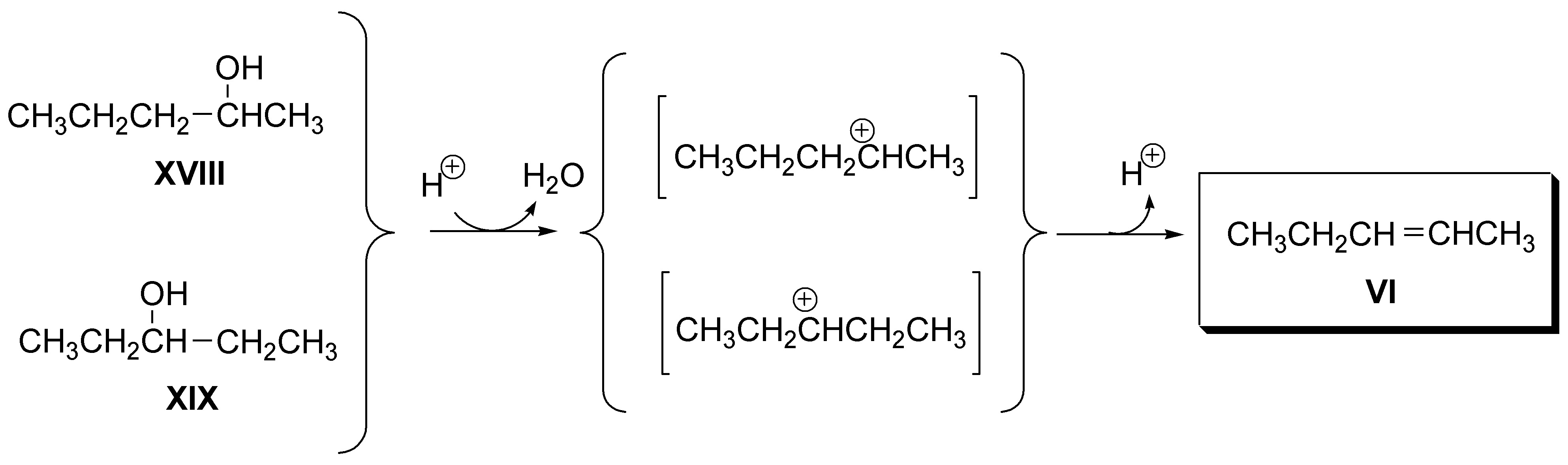
Solution 9:
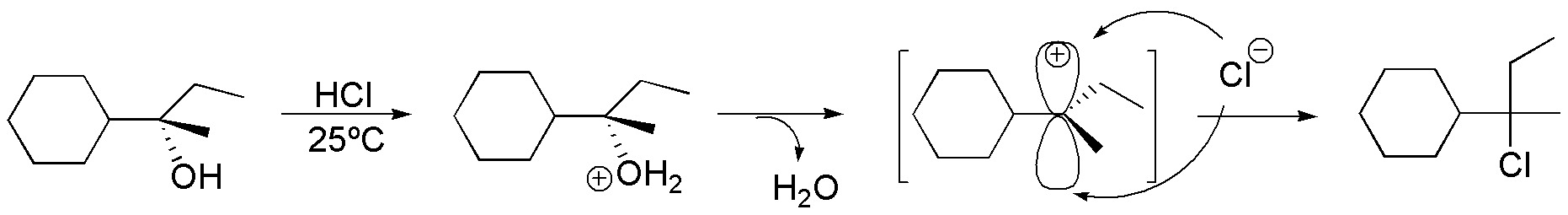
(a) The starting product of the reaction is a chiral tertiary alcohol. By treatment in acid medium a tertiary carbocation is formed. The carbocations are reaction intermediates which can be attacked by nucleophiles on both sides, resulting in an equimolecular mixture of the two possible enantiomers (racemization). In our case the nucleophile is the chloride ion and the final product of the reaction is a racemic tertiary alkyl chloride.
b) The reaction of an alcohol with SOCl2 leads to the formation of alkyl chlorides, which in the absence of a base takes place by an intramolecular mechanism (SNi). As a result an alkyl chloride with retention of the configuration is obtained.
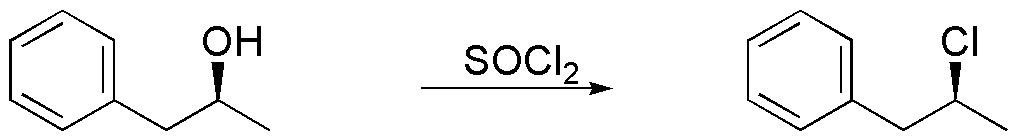
c) Treatment of a primary alcohol such as pentan-1-ol with phosphorus trichloride results in the substitution of the hydroxyl group by the halogen atom. The mechanism of this reaction is of the SN2 type, which means that it takes place with inversion of the configuration, although in this substrate, since the carbon on which the reaction takes place is achiral, no stereochemical changes are observed.
![]()
d) The reaction described consists of two steps, in the first one the primary alcohol is transformed into the corresponding mesylate (methanesulfonyl derivative) by treatment with mesyl chloride in basic pyridine medium. The mesylate, like the other sulfonates, is an excellent leaving group which can be substituted with hydrogen by an SN2-type reaction in which a hydride of LiAlH4 acts as a nucleophile. The reaction can be considered overall a reduction in that globally there is loss of oxygen and gain of hydrogen.
![]()
Solution 10:
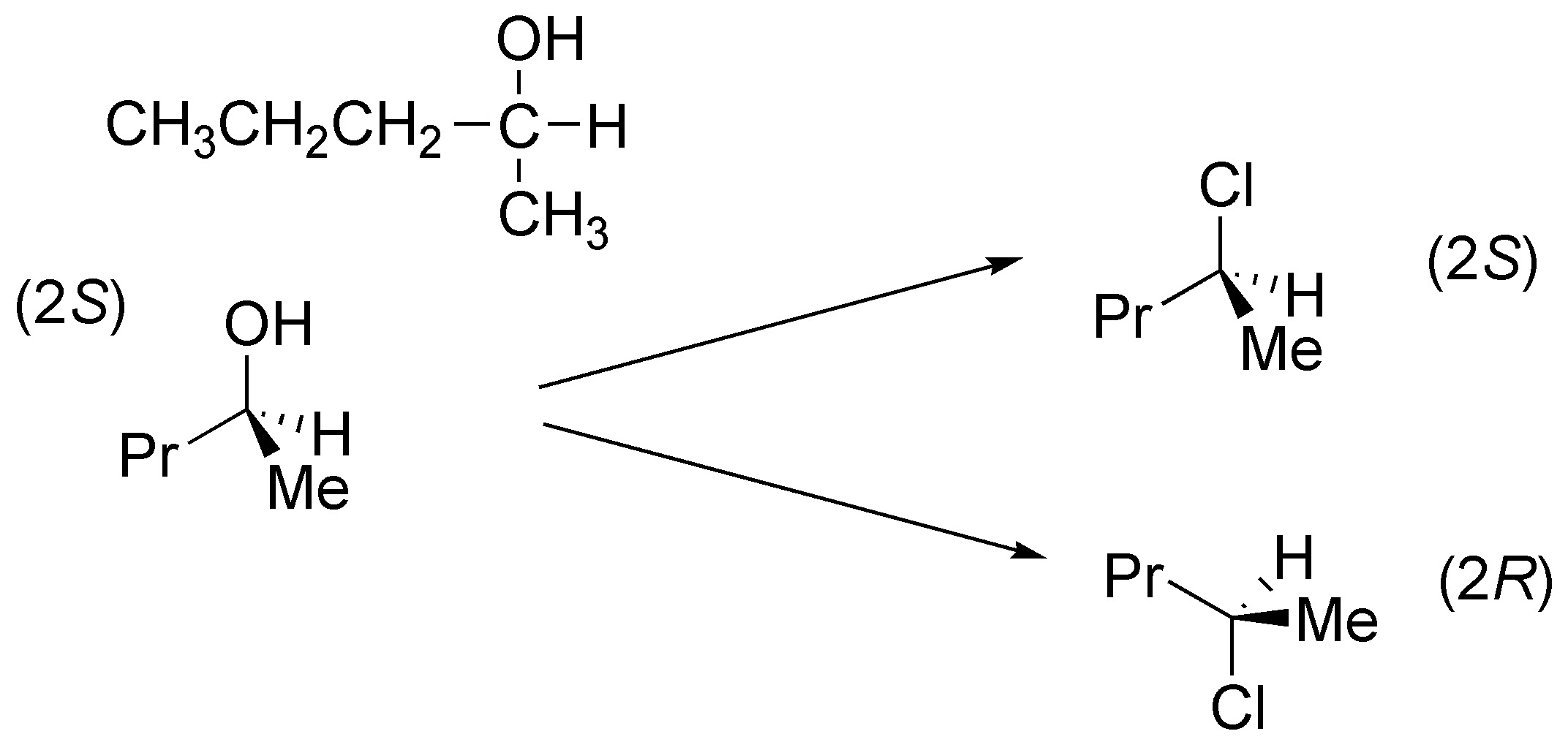
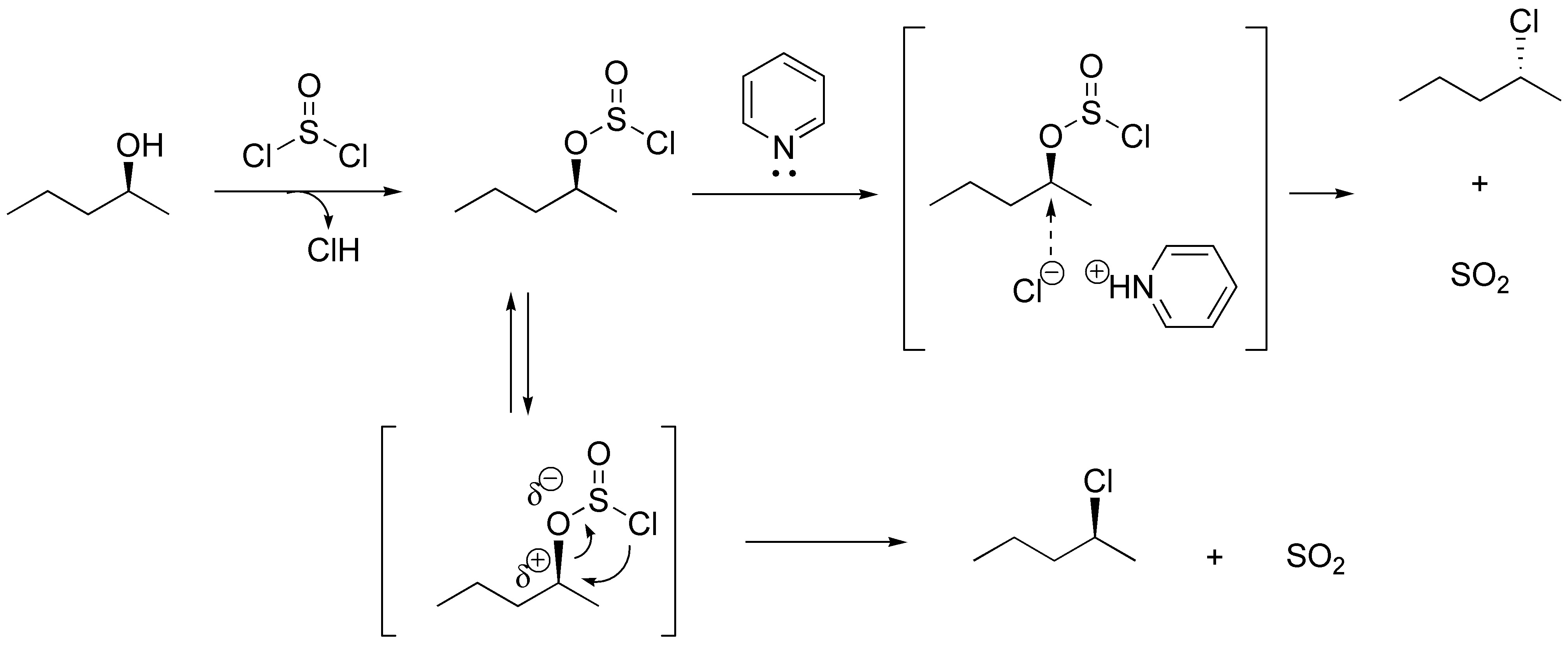
Both reactions can be performed with the same reagent thionyl chloride (SOCl2) if the reaction is performed in the presence of pyridine there is conversion of the alcohol to halide via an SN2 and thus configuration inversion occurs. If performed in the absence of base the absolute configuration is maintained by an intramolecular process.
Solution 11:
(a) Cyclohexanol is a secondary alcohol, which by oxidation gives a ketone. Reagents that can give this transformation are: PCC (ClCrO3 • C5H5NH), Collins’ reagent, CrO3 • 2 C5H5N, Jones’ reagent, CrO3 • H2SO4, Swern’s reagent, (CrOCl)2/ DMSO, and potassium permanganate.

b) Cyclopentylmethanol can be transformed into cyclopentanecarboxylic acid by treatment with potassium permanganate or with Jones reagent, CrO3 / H2SO4.
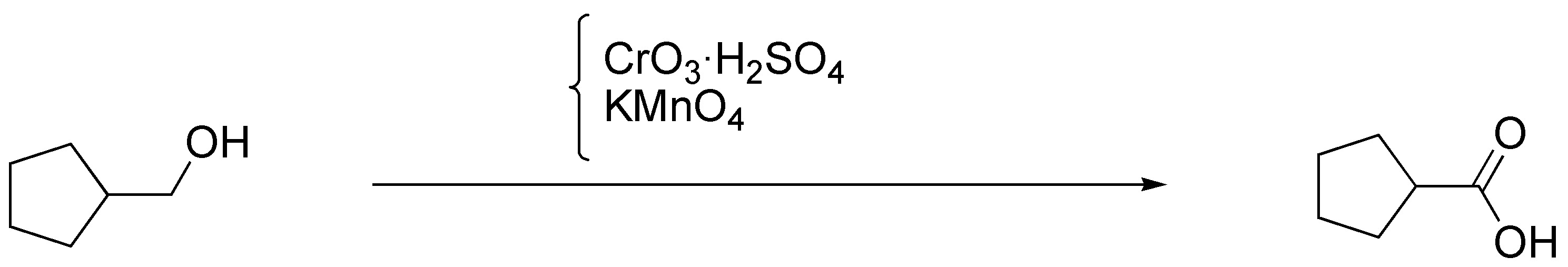
c) On the same substrate of the previous exercise it is necessary to use a milder oxidant to obtain the aldehyde and that this one is not transformed into carboxylic acid. The reagents used for this purpose are: PCC (ClCrO3 • C5H5NH), Collins’ reagent, CrO3 • 2 C5H5N, and Swern’s reagent, (CrOCl)2 / DMSO.

d) For the transformation of 2-methylpent-4-en-1-ol to 2-methylpent-4-enal any of the procedures in the previous section can be used, since in no case will the double bond be affected, inasmuch as there is no protic acid in any of the reagents described.

Solution 12:
(a) The starting product of the exercise is a primary alcohol. With PCC the corresponding aldehyde with the same carbon skeleton will be obtained. Under the reaction conditions the double bond is not affected.
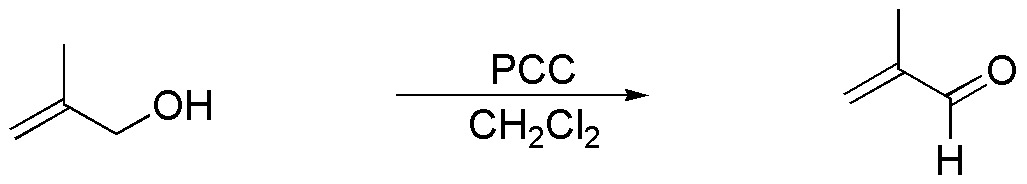
b) Lithium aluminum hydride is a reductant which does not affect the double bond. Because of its strongly basic character it can generate the corresponding alkoxide. If the reaction is treated with aqueous ammonium chloride, as a weak acid, it neutralizes the basicity of the medium, so that no reaction will be observed.
c) The reaction of an alcohol with thionyl chloride in pyridine produces the substitution of the hydroxyl group by a chlorine atom. The reaction proceeds by an SN2 mechanism. Being a substrate where the -OH group is in a primary position, from the stereochemical point of view, no consequence is observed due to the inversion at the carbon attached to the -OH group.
![]()
d) Jones’ reagent is an oxidant capable of oxidizing primary alcohols to carboxylic acids and secondary alcohols to ketones. However, in this case the reagent is incompatible with the starting compound, since the acidic medium affects the double bond.e) The reaction described is an esterification reaction, using benzoyl chloride as an acylating agent. The result is a benzoate. The presence of a base is required for the reaction to occur.
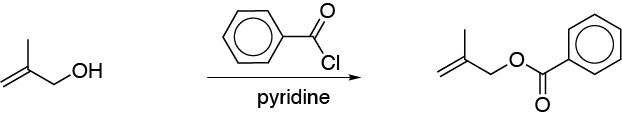
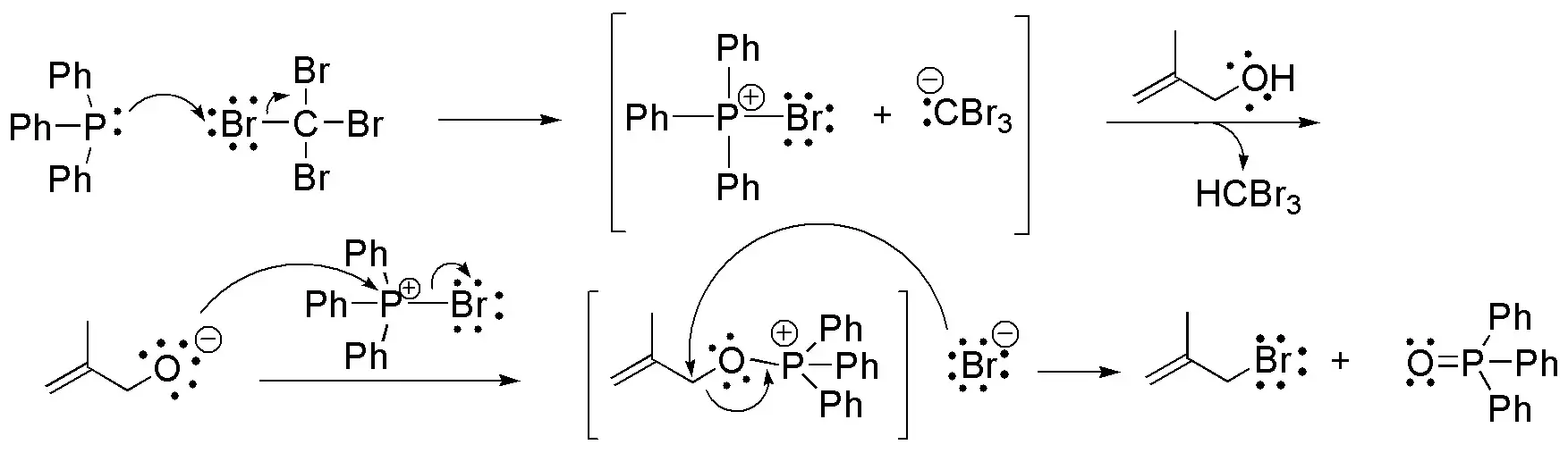
f) The Appel reaction involves the substitution of a hydroxyl group for a bromine atom using triphenylphosphine and tetrabromine (Br4C). The reaction conditions are very mild, and usually show very good yields.
![]()
It is known that hydroxyl groups are very bad leaving groups in SN2-type reactions, so they cannot be substituted by a nucleophile directly. It is necessary to form a derivative, usually sulfonates, to perform the substitution. In this case, the nucleophile (X-) is generated in situ, at the same time as the triphenylphosphine associates with the -OH group to convert it into a good leaving group. Triphenylphosphine oxide is produced as a byproduct of the reaction.
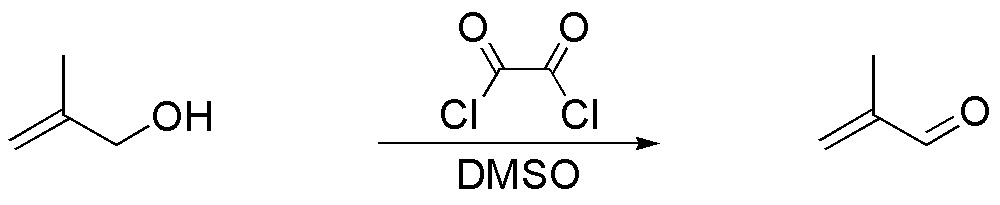
g) The oxalyl chloride / DMSO system is called Swern’s reagent and allows the oxidation of primary and secondary alcohols to the corresponding carbonyl compound. The primary alcohol in the exercise gives the same compound as in the previous section, since the double bond is not affected.
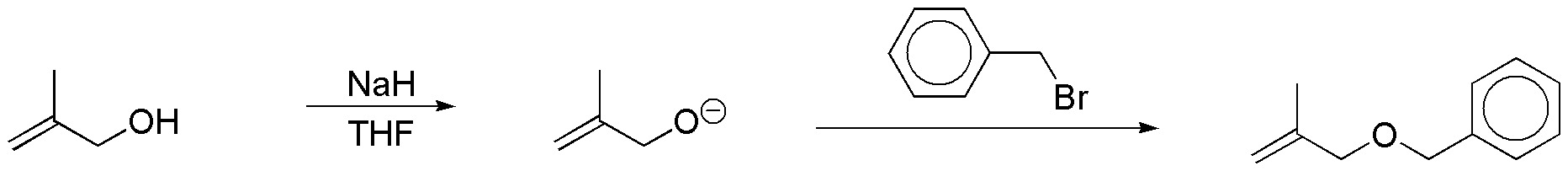
h) Sodium hydride is a strong base that reacts with alcohols to give alkoxides. Sodium hydride is incompatible with protic solvents such as water or alcohols, so the reaction must be carried out in aprotic solvents such as THF, which, as is known, is a cyclic ether. Alkoxides are nucleophiles that can displace halogen atoms in primary and secondary halides by SN2-type reactions. This procedure is a general method for the preparation of ethers and is called Williamson synthesis. Applied to the substrate of the problem the sequence of reactions is as follows:
Solution 13:
The di-n-propyl ether (A) will be treated. The treatment of 1-propanol with sodium allows the formation of sodium alkoxide (eliminating H2), this alkoxide will react with the primary alkyl halide via a SN2 (Williamson Synthesis) producing an ether.
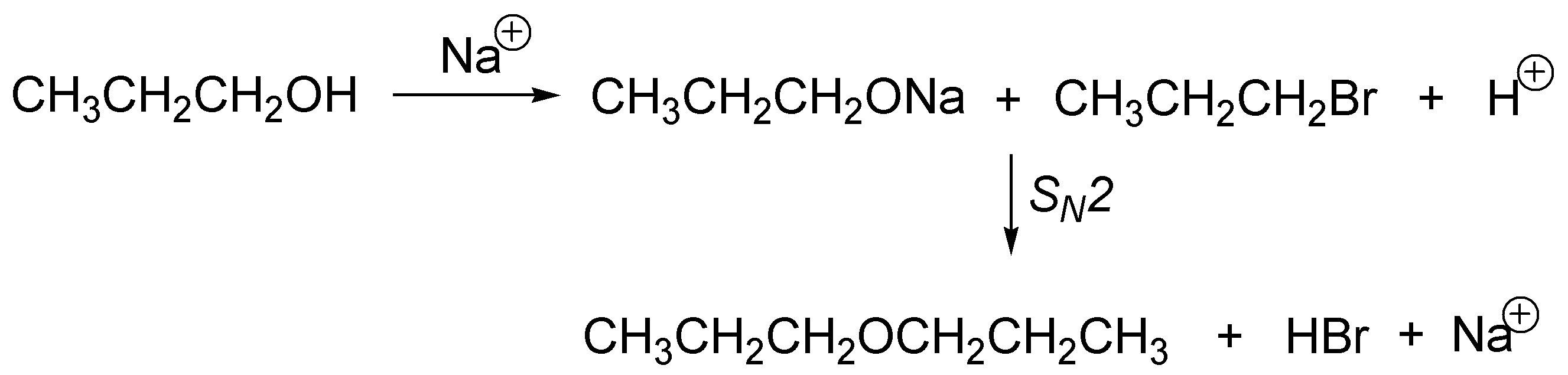
Solution 14:
The Williamson synthesis of ethers consists of SN2 type substitution on an alkyl halide with an alkoxide acting as a nucleophile. The order of reactivity on halides, as is known, is:
primary > secondary >> tertiary
Because bromine is a better leaving group than chlorine, and in general, bromides are more stable than iodides, primary or at most secondary alkyl bromides of suitable structure should be chosen for the syntheses of the exercise compounds. Applying these principles for compounds a), b) and c) we have the following results:
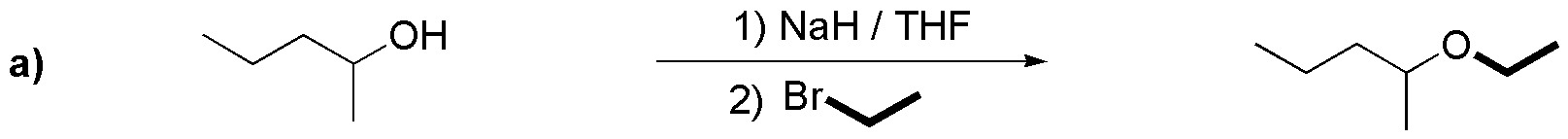
(b) In this case, alkyl bromide and alcohol can be chosen indifferently on one or the other fragment, since in both cases we start from a substrate with the -OH or -X group on a primary carbon.

c) For this third compound the bromide chosen must be methyl bromide bromide and as alkoxide tert-butoxide, according to the following scheme:

Solution 15:
The only possibility is oxacyclopropane (ethylene oxide).
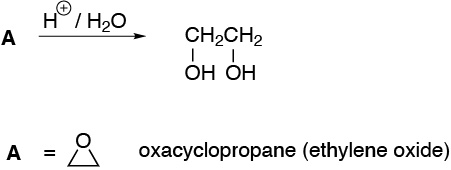
Solution 16:
As seen in the following scheme, the only solution is 2,3-dimethyloxycyclopropane. The problem would be much more complex if the stereochemistry of the products formed were indicated.
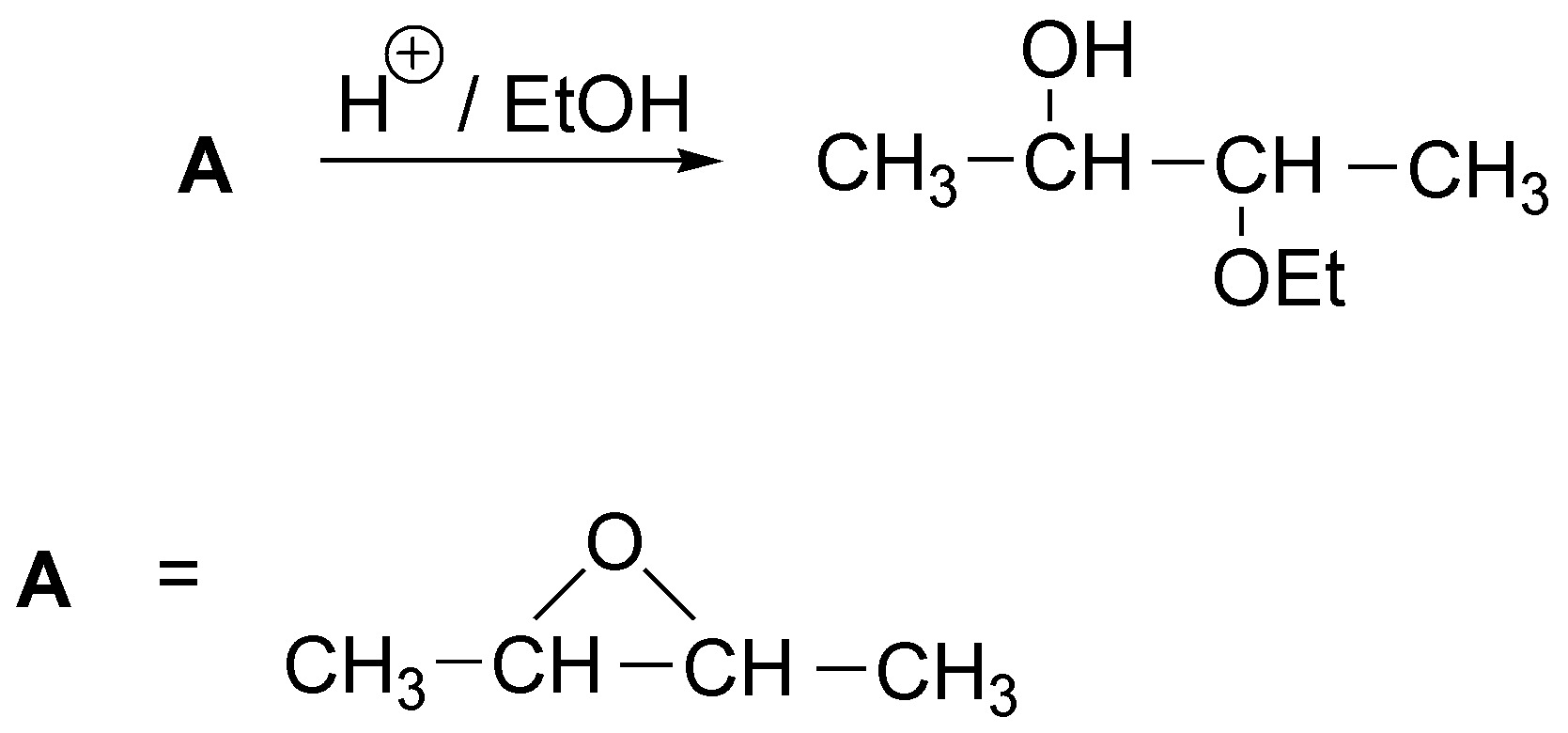
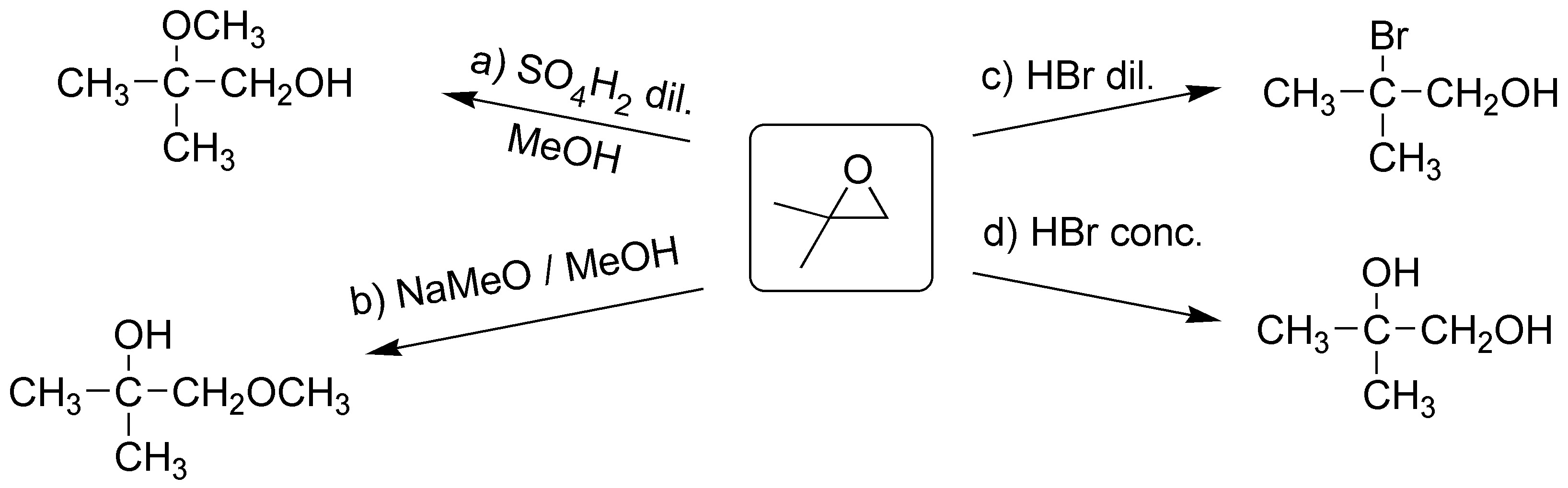
Solution 17:
(a) Epoxides in acidic media open so that the nucleophile, which can be the solvent itself as in this case, attacks the most substituted carbon, since it is in this position that the highest positive charge density occurs. The nucleophile remains in the anti position, with respect to the hydroxyl that is generated from the oxygen atom of the epoxide. On the substrate of exercise a), the two carbons supporting the oxirane ring are equally substituted, so there is no preference in the attack of the nucleophile (water).
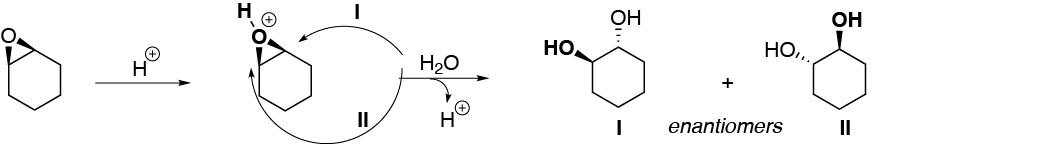
b) In this second case, the epoxide is not symmetrically substituted, so the attack of the water molecule occurs on the most substituted carbon, since the presence of a methyl group stabilizes the positive charge on this carbon.
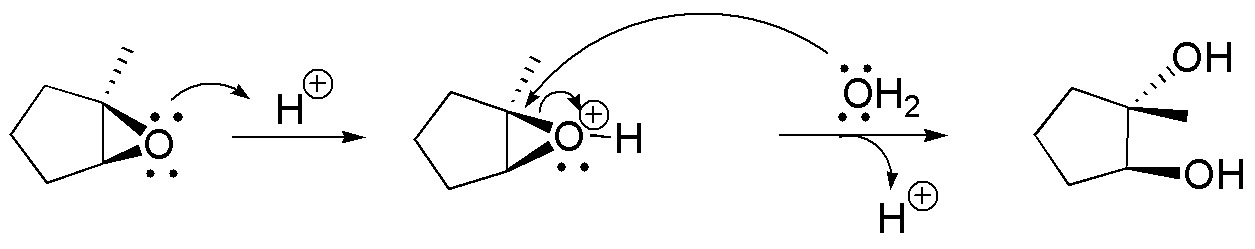
c) Grignard’s reagent has a carbon with nucleophilic character that will attack the less substituted carbon of the epoxide, obtaining a product, according to the scheme:
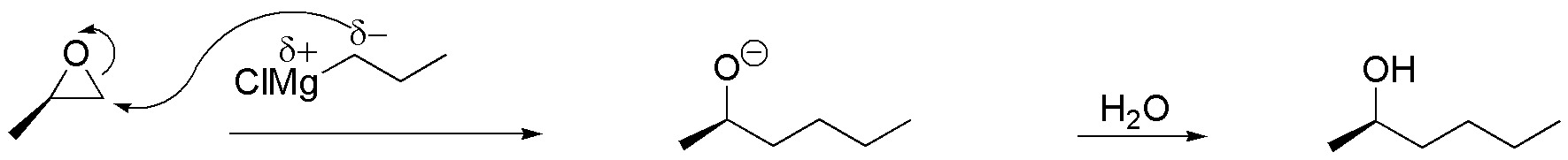
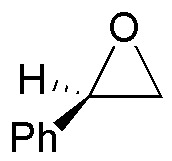
Solution 18:
(a) The compound comes from the opening of an epoxide with azide, which is a fairly good nucleophile. Since the azide is located on a primary carbon, and because of the arrangement of the hydroxyl group, the starting epoxide must be:
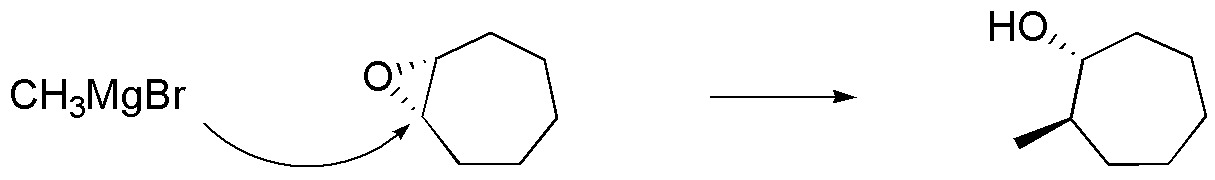
(b) In this compound a new C-C bond is generated, so the nucleophile used must be an organometallic compound, such as methylmagnesium bromide. The attack of the nucleophile occurs on the opposite side to where the epoxide is located. The indicated product is accompanied by its enantiomer, since the attack of the nucleophile is just as likely on the two carbons forming part of the three-membered ring.
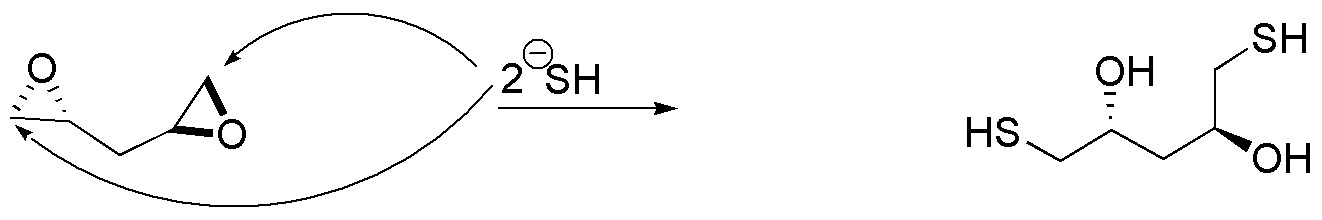
c) The described product comes from the simultaneous opening of two epoxides located at the end of a carbon chain, with the bisulfide ion as nucleophile. This nucleophile binds to the least substituted carbon, which in both cases is the primary carbon. The stereochemistry of the starting epoxides can be deduced based on the hydroxyl configuration of the final product.
d) This compound is prepared from an epoxide with methanol in an acidic medium. Methanol acts as a nucleophile by attacking the most substituted carbon, because it has the highest positive charge density.

Solution 19:
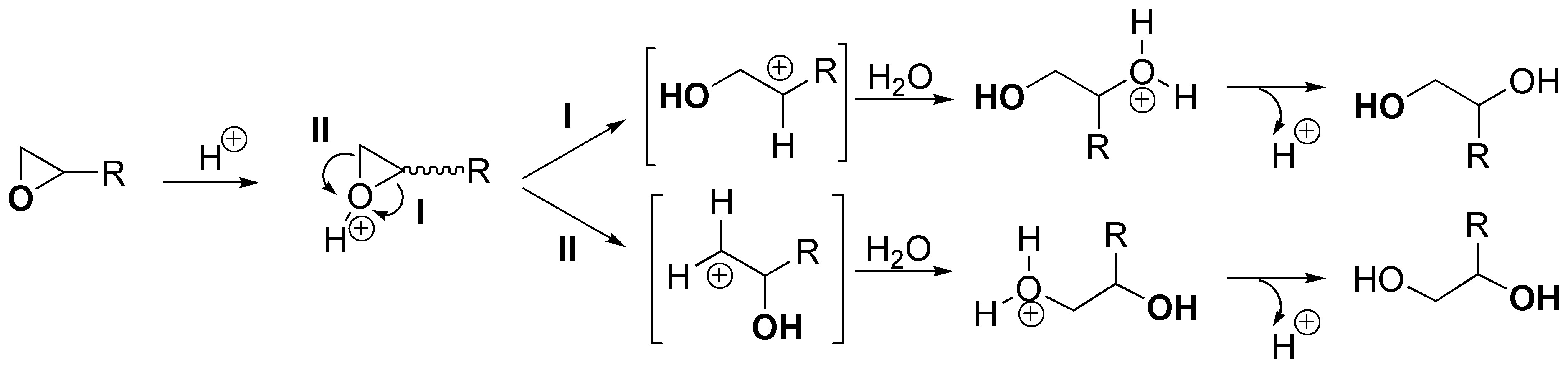
The general scheme of the process described would be as follows: protonation of oxygen occurs producing a cyclic ion that can evolve by breaking a C-O bond by route I or by route II, when attacked by a nucleophile such as water. Route I is more favored than route II, since on the most substituted carbon the positive partial charge that is generated as the C-O bond is broken is more stabilized.
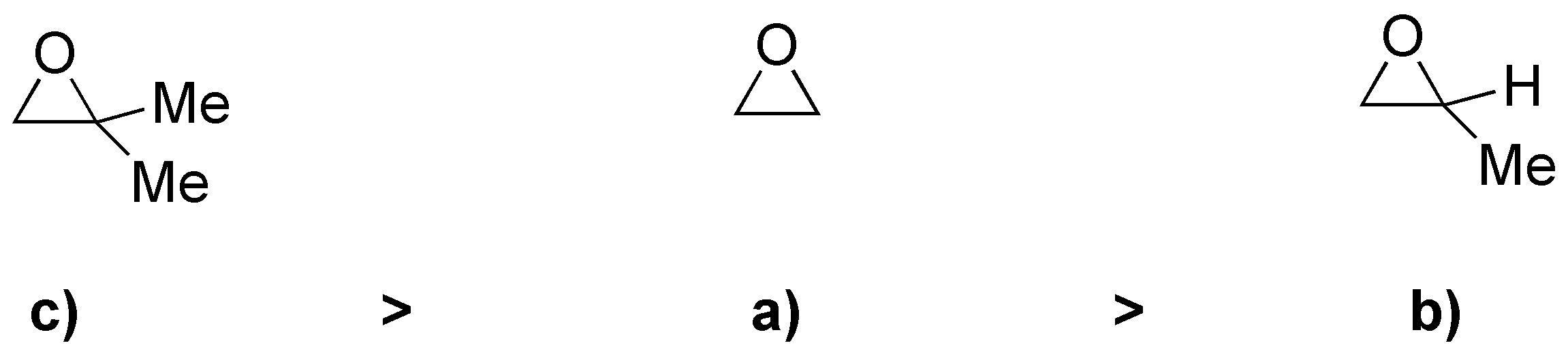
On the other hand, the greater the capacity of R to stabilize the positive charge, the more favored will be the process, and therefore the greater will be the reaction rate. With all these considerations the rate of the reaction for the different substrates will be:
Solution 20:
This problem deals with the opening of epoxides (oxacyclopropanes). Nucleophilic opening occurs by attack on the least hindered carbon: cases a), c) and d); whereas acid catalyzed opening causes the nucleophile to attack the most substituted carbon: b).
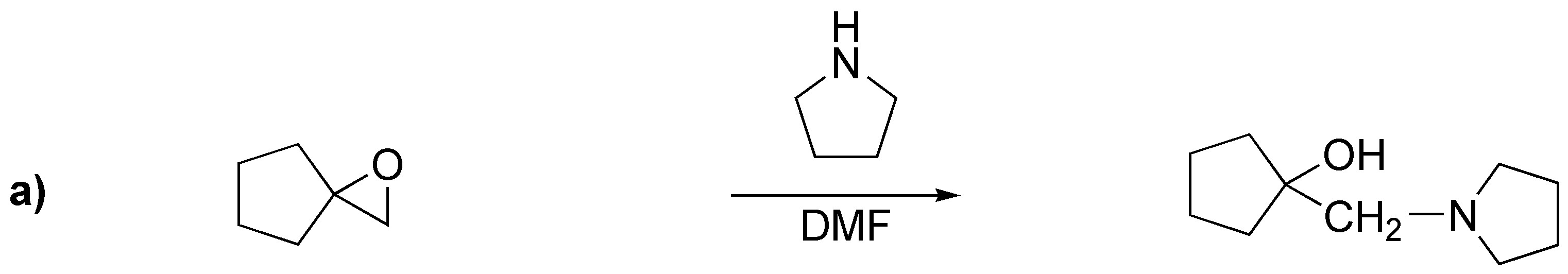
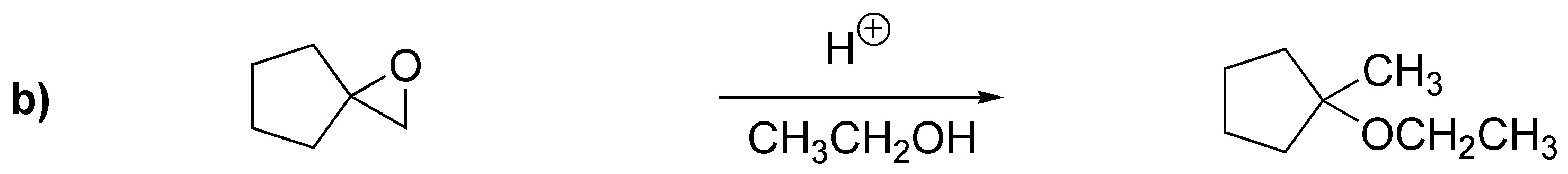
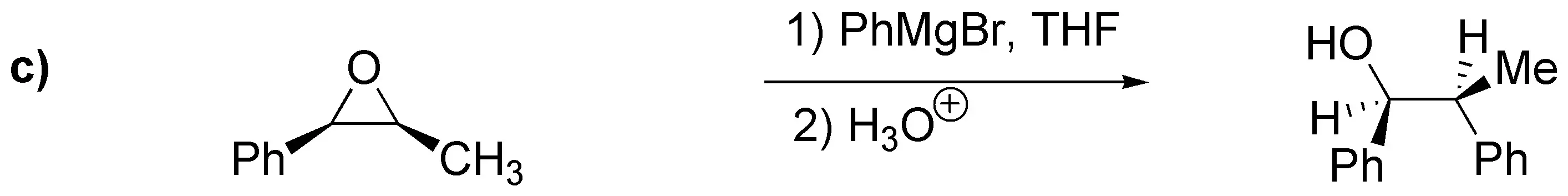
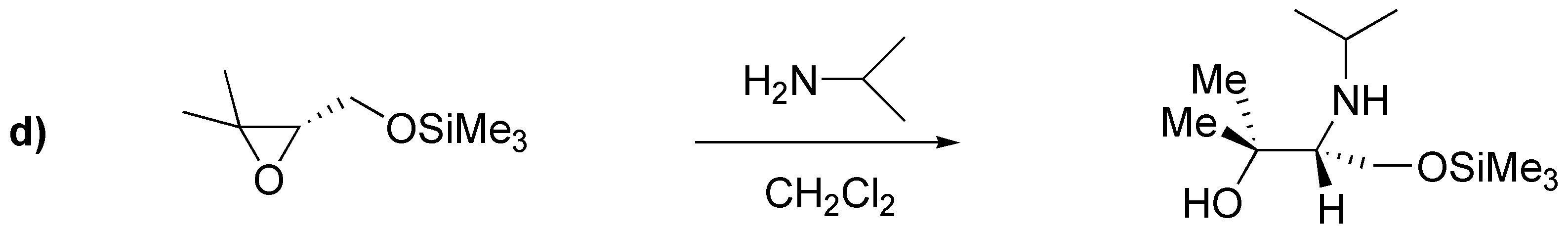
Solution 21:
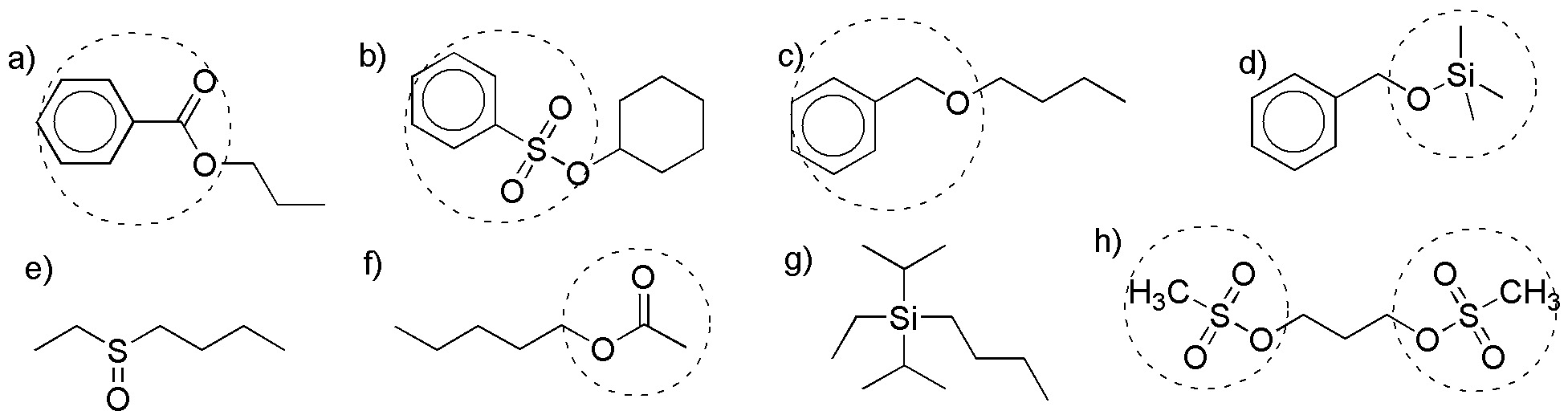
a) ester: benzoate; (b) sulfonate: benzenesulfonate; (c) ether: benzyl ether.
d) silyl ether: trimethylsilyl ether; e) sulfoxide (not an alcohol derivative); f) ester: acetate; h) sulfonate: mesylate.
Solution 22:
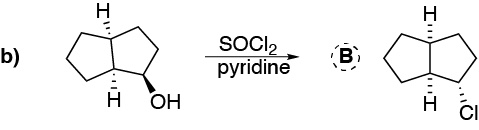
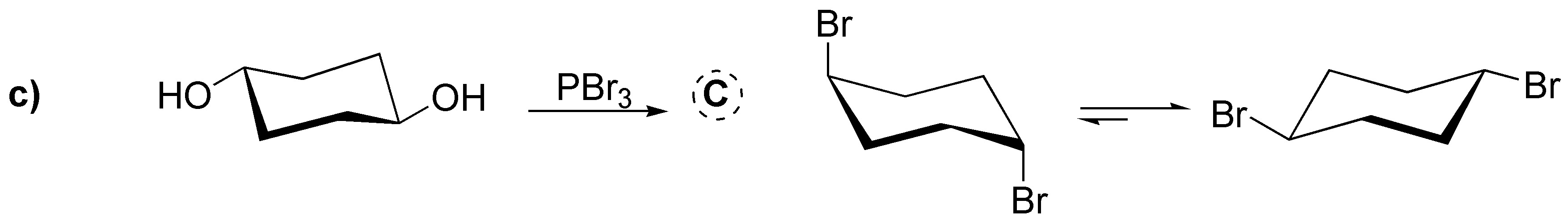
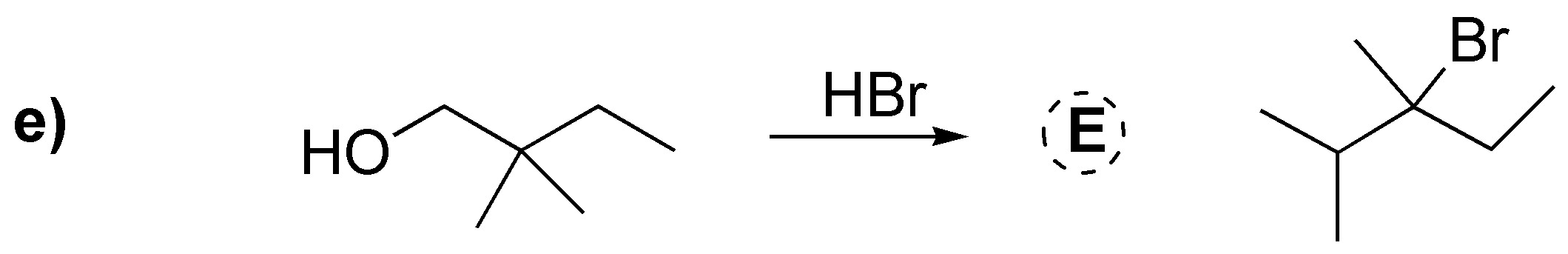
We deal in this problem with the conversion of alcohols to haloalkanes by various procedures: a) Treatment with HCl produces the corresponding chloride; b) With thionyl chloride the corresponding chloride is produced with inversion of the configuration, as in c) gives the corresponding dibromo derivative trans from the dialcohol trans (stereospecific reaction). In d) conversion to iodide occurs with inversion of the configuration after transformation of the hydroxyl into a tosylate which is the better leaving group. In e) protonation of the hydroxyl group and subsequent dehydration would generate a primary carbocation that undergoes a transposition to a tertiary carbocation by migration of a methyl group; this carbocation is trapped by the bromide ion.
![]()

Solution 23:
Dehydration of alcohols fulfills Zaitsev’s rule by producing the most substituted alkene:
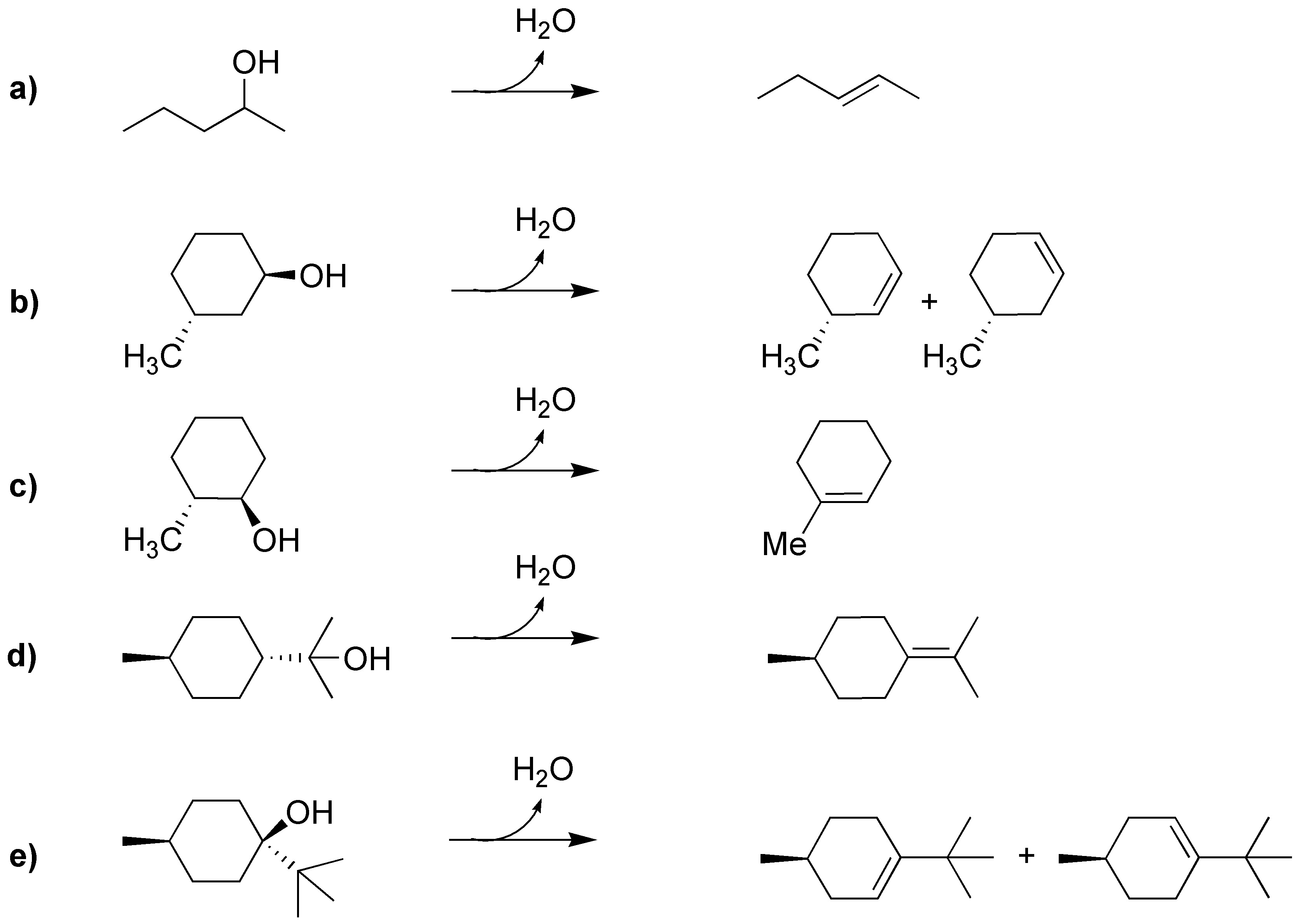
Solution 24:
The solution is usually not unique: several alcohols can give the same alkene, as we see in the scheme:
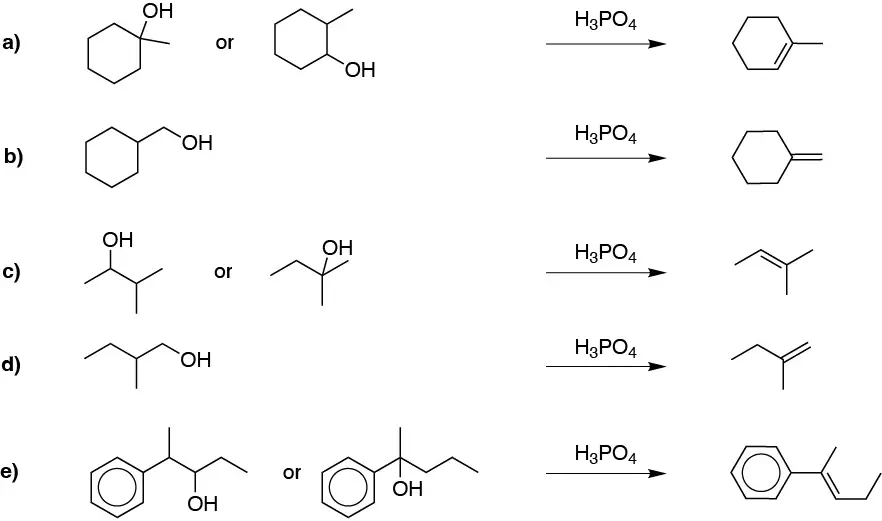
Solution 25:
When an alcohol is treated with thionyl chloride it is transformed into a chloroalkane; when treated with PCC it is oxidized to the corresponding carbonyl group, in this case to ketone and finally the alcohols react with sodium forming the alkoxide or alcoholate which acts as a nucleophile against haloalkanes giving ethers:
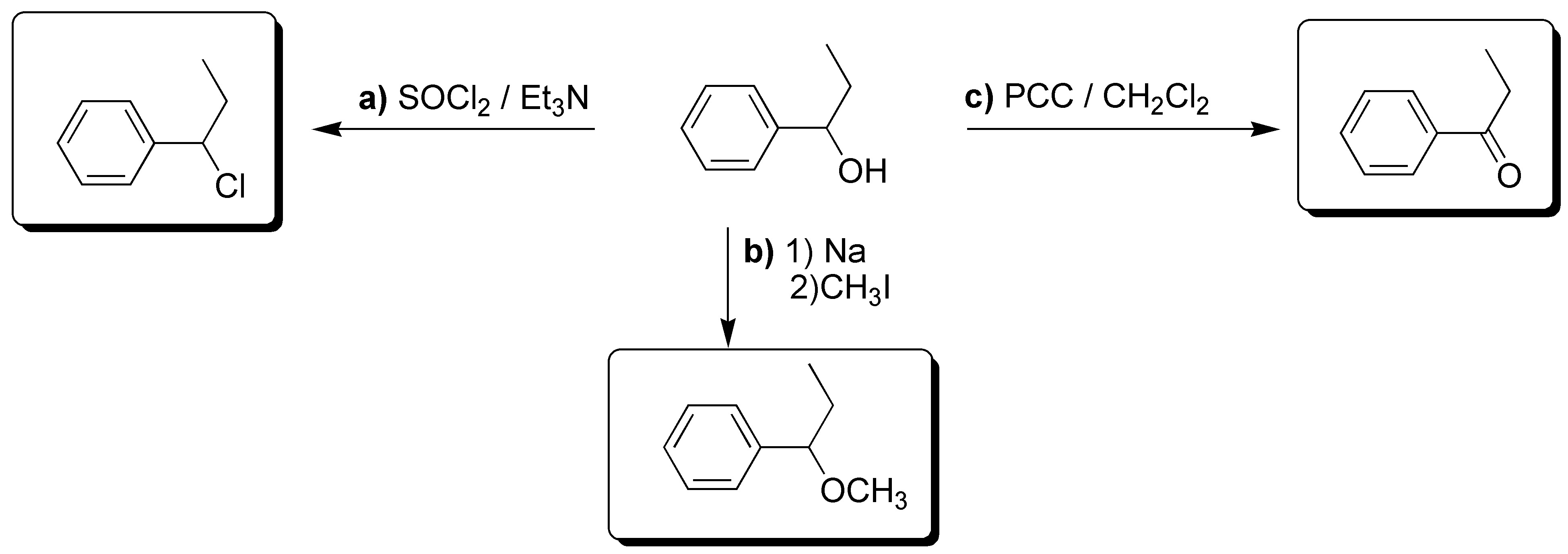
Solution 26:
The simplest explanation is as shown in the scheme. The instability of the cyclobutane ring causes transposition to occur generating the cyclopentyl cation which is trapped by the bromide ion:
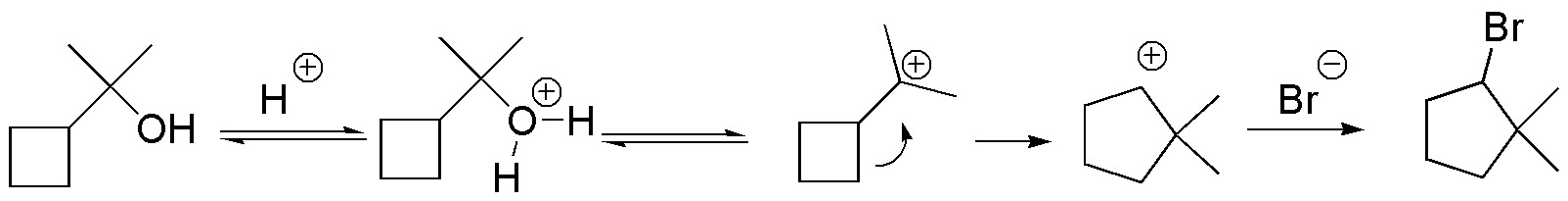
Solution 27:
We review with this scheme the oxidation reactions of alcohols according to their nature. Primary alcohols are oxidized to aldehydes with mild oxidants such as PCC or to acids with strong oxidants, secondary alcohols to carbonyl compounds and tertiary alcohols are not oxidized. Alkylbenzene compounds oxidize to benzoyl acids.
| Compound | PCC | Jones’ reagent | Swern’s reagent | KMnO4 |
 | A
| B
| C
| D
|
| E No reaction | F No reaction | G No reaction | H No reaction | |
I | J Mixtures, double bond acid-sensitive | K | L |
Solution 28:
(a) alcohols can be converted to chloroalkanes by the action of HCl or thionyl chloride (SOCl2). b) Oxidation to carboxylic acids can be achieved by Jones’ reagent (CrO3 / H2SO4). c) One of the methods of converting alcohols to bromoalkanes is the Appel reaction (CCl4/PPh3). d) To convert an alcohol to an ether a good solution is the Williamson synthesis (treatment of the alcoholate with haloalkane) we should try to use a primary haloalkane otherwise we would also obtain elimination product. e) The conversion of alcohol to alkane can be done by sulfonation of the alcohol and reduction of this with LiAlH4. f) The conversion to a Grignard compound (alkylmagnesium chloride) will be done after conversion of the alcohol to chloroalkane.
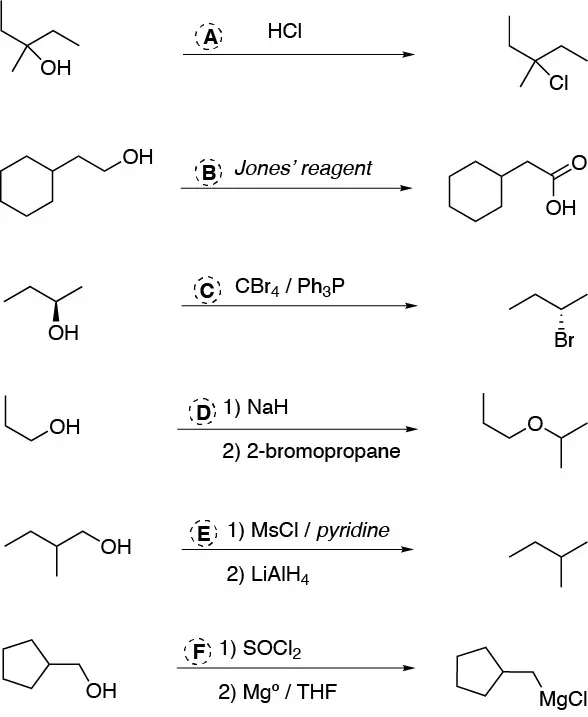
Solution 29:
The reaction of the ethers with HI (very strong acid) produces an alkoxonium ion, this ion is nucleophilically attacked by the iodide ion on the least hindered carbon producing an alcohol and an iodoalkane:
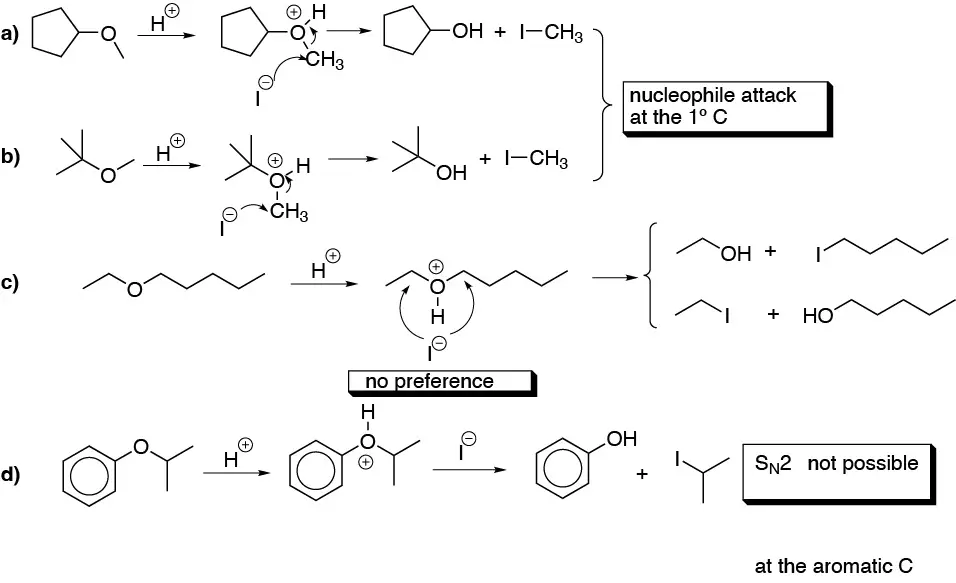
Note that in b) if an excess of HI is used, in all the alcohols obtained in the step described above, substitution of the -OH group by -I occurs.
R-O-R’ + IH(excess) → R-I + R’-I
Solution 30:
This problem deals with the opening of epoxides (oxacyclopropanes). Nucleophilic opening occurs by attack on the least hindered carbon; whereas acid-catalyzed opening causes the nucleophile to attack the most substituted carbon:
Solution 31:
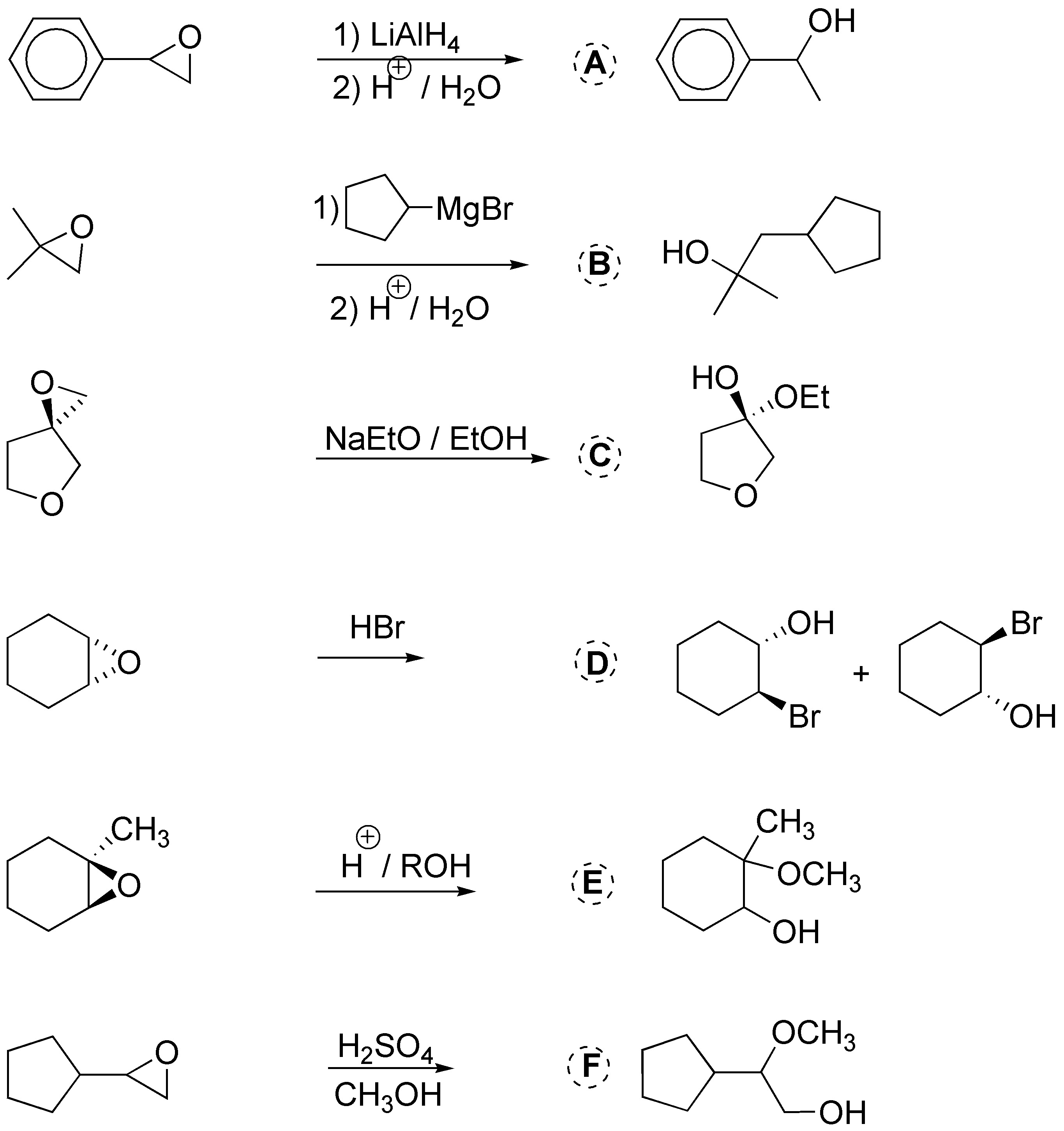
We review some reactions of oxiranes (epoxides, oxacyclopropanes); in A and B we obtain alcohols as a consequence of opening with magnesians, in C with acetylide and in D with a thiolate, in the latter case the opening is accompanied by a nucleophilic substitution of chlorine.
Solution 32:
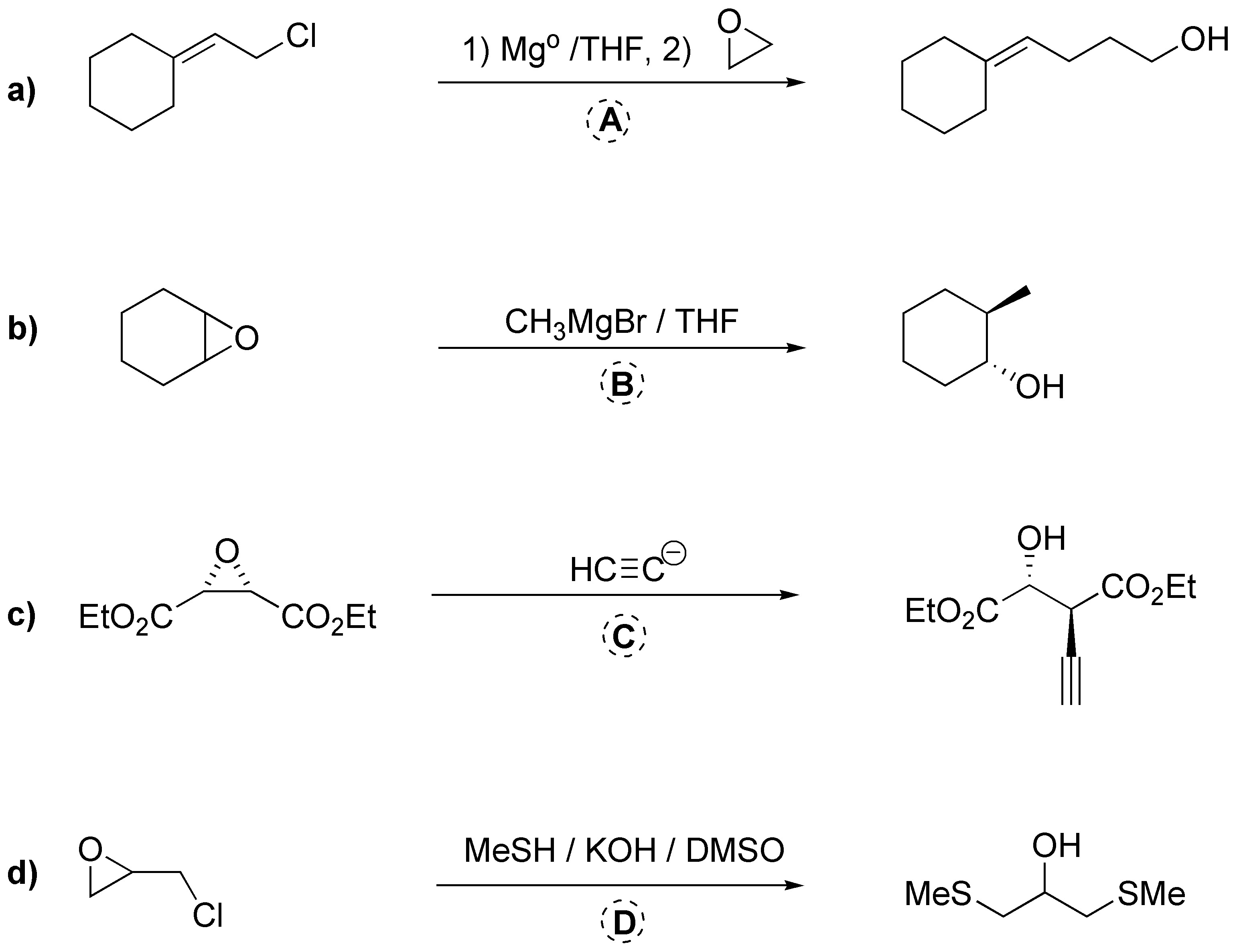
In case a) the iodine is added to the double bond generating a cyclic iodonium ion which is attacked by the hydroxyl (nucleophilic) group in an intramolecular reaction giving the expected iodoalcohol. In b) the amine acts as a nucleophile opening the epoxide and subsequently the aminoalcohol obtained produces a new substitution reaction, generating the final aziridine.
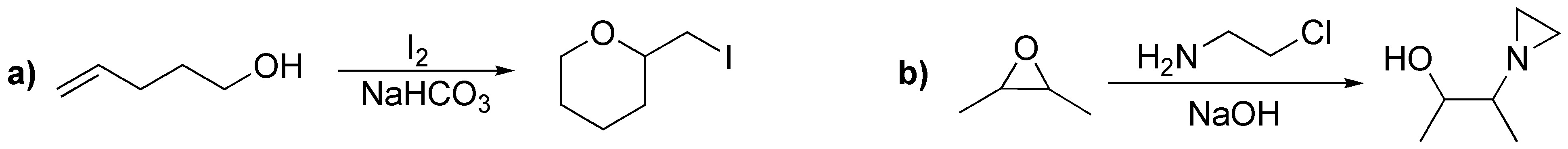
Solution 33:
The reaction in the first column is a reduction of the epoxides to alcohols (nucleophilic addition of the hydride ion). The second is the nucleophilic addition of an organometallic (organocuprate). The third and fourth are two acid-catalyzed epoxide openings in which the nucleophiles are water and ethanol.
| Substrate | 1) LiAlH4 ; 2) H3O+ | (Et)2CuLi | H2O / H3PO4 | CH3CH2OH / H3PO4 |
|---|---|---|---|---|
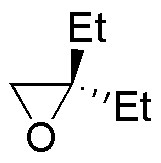 | A
| B
| C
| D
|
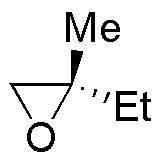 | E
| F
| G
| H
|
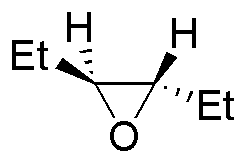 | I
| J
| K
| L
|
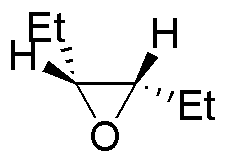 | M
| N
| O
| P
|
 | Q
| R
| S
| T
|
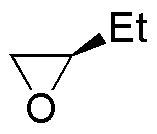
Solution 34:
Let’s review some of the reactions of alcohols:
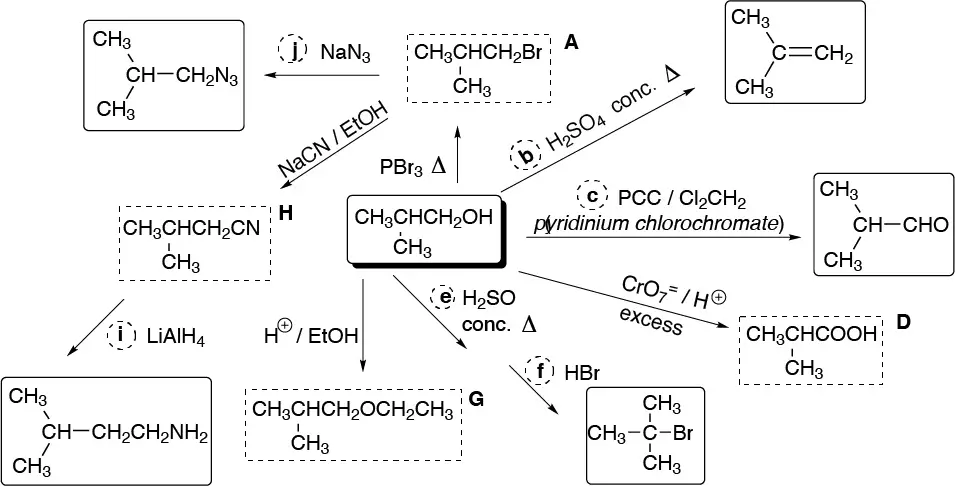
Solution 35:
The problem illustrates the two possibilities for opening epoxides: in an acidic medium or by means of a nucleophile:

Solution 36:
The scheme shows general reactions of alcohols. a) alcoholate or alkoxide formation; b), c) and d) conversion to haloalkane, e) elimination and f) ether formation.
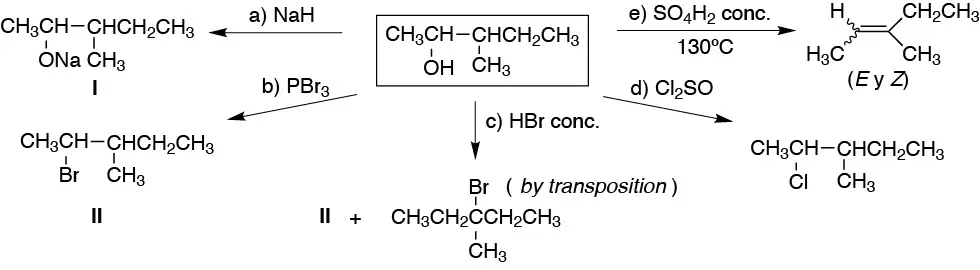
Solution 37:
The scheme illustrates the possible openings of epoxides (oxacyclopropanes) in both acidic and basic media: