Written by J.A Dobado | Last Updated on April 22, 2024
Go to the page with the list of problems.
Enolates – solutions to problems
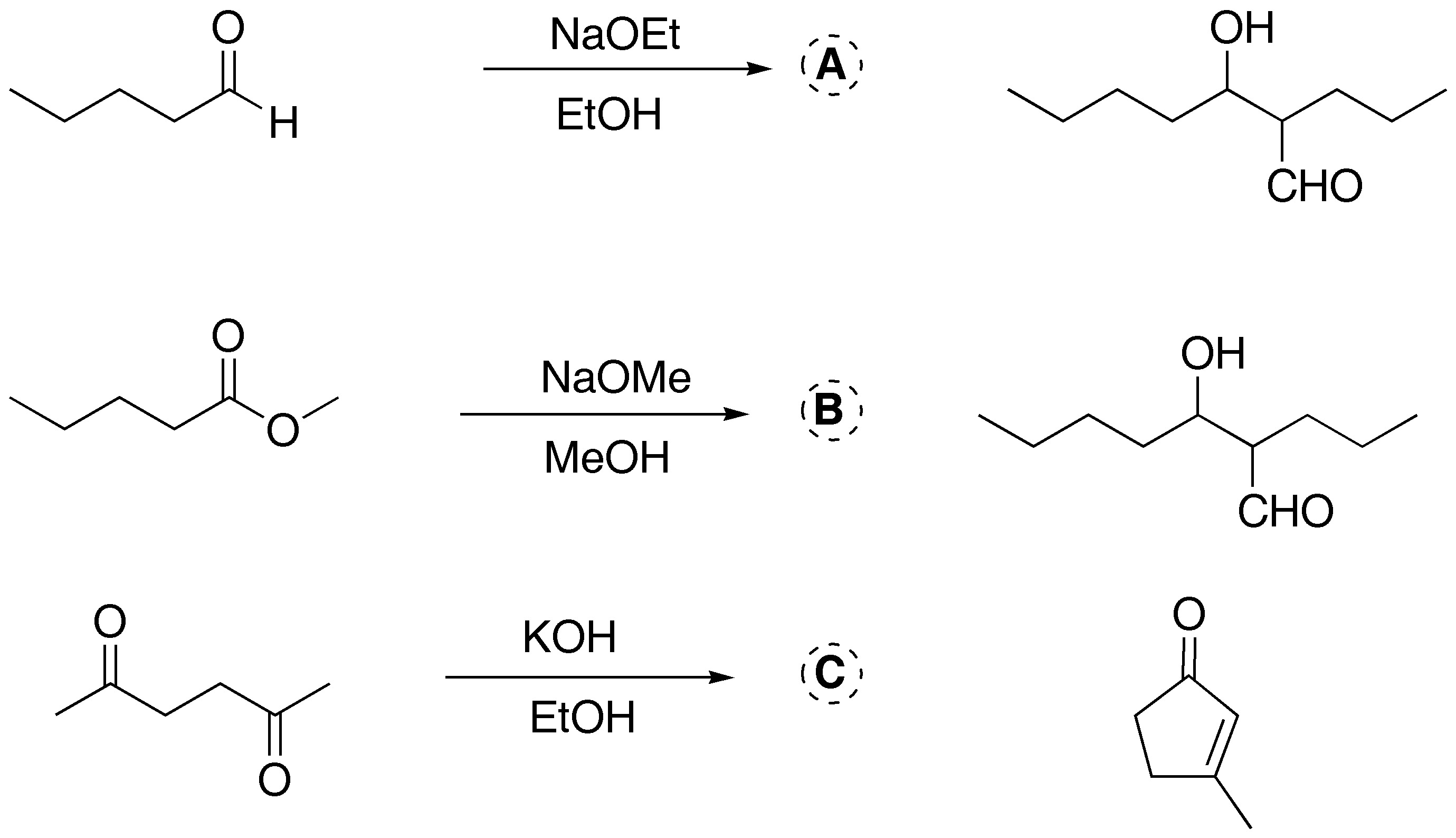
Solution 1:
The first two exercises correspond to an aldol condensation and Claisen condensation, respectively, giving rise to the same compound. In case of high temperature or traces of acid, the elimination of a water molecule and the formation of a conjugated double bond with the carbonyl group would occur. In the last exercise of this problem, intramolecular cyclization occurs, especially favored by the formation of a five-membered ring.
Solution 2:
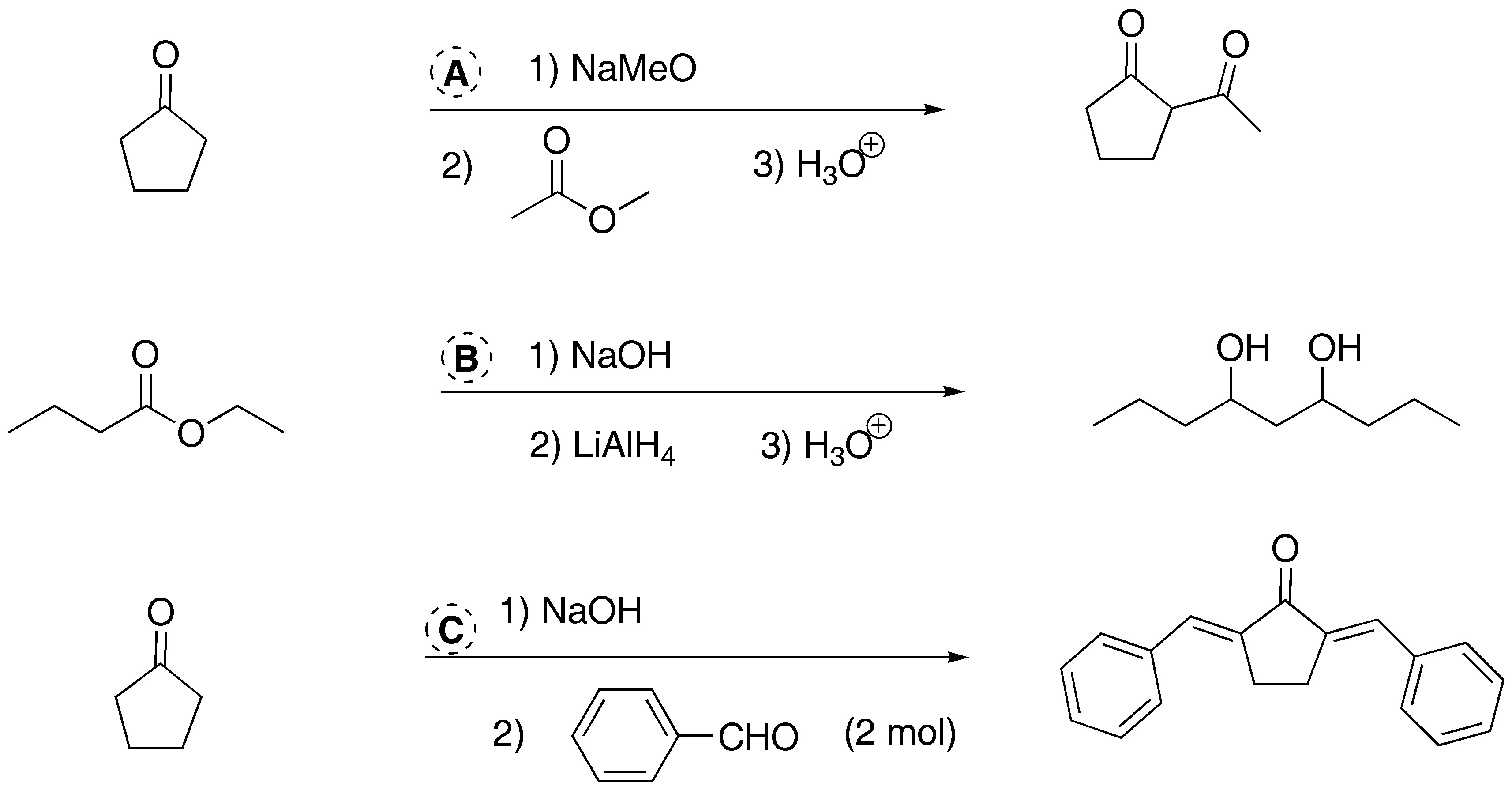
a) Treatment of cyclopentanone with sodium methoxide generates a carbanion that reacts with ethyl acetate. Because the two positions adjacent to the carbonyl group are equivalent, a single product is obtained.
b) This is a typical Claisen condensation, which would give a dicarbonyl compound. Treatment with LiAlH4 leads to the formation of a diol. It is necessary to add an acid to destroy the alkoxides formed in the course of the reaction, due to the pronounced basicity of the hydride.
c) Cyclopentanone treated with an excess of base can give a carbanion on both sides of the carbonyl group. By reaction with two moles of benzaldehyde the final product of the reaction is obtained.
Solution 3:
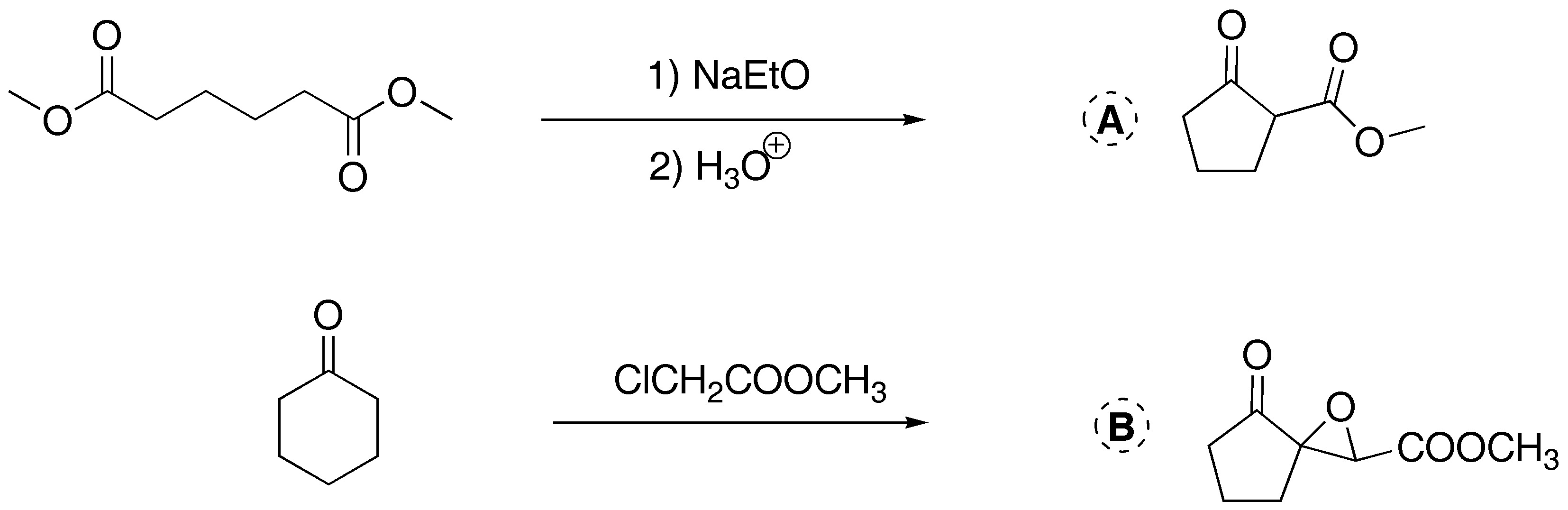
a) Treatment with a base of the diester leads to the formation of a cyclic compound. This is a Dieckmann condensation (intramolecular Claisen).
b) The cyclohexanone forms a carbanion which reacts with the α-haloester. The intermediate alkoxide displaces the chlorine by an SNi process to give the α,β-epoxy ester.
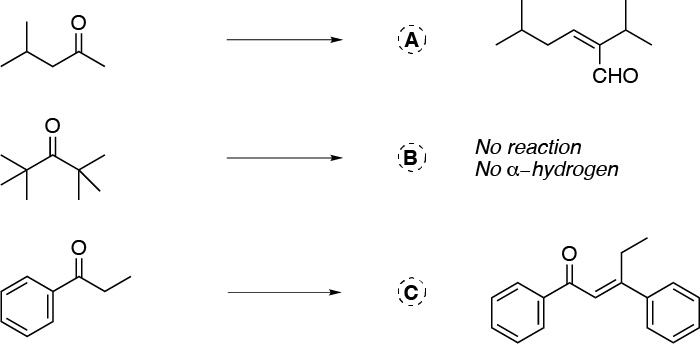
Solution 4:
Reactions A and C are aldol-type condensations. For them to occur, the use of a base such as alcoholic potash, a mixture of potassium hydroxide and ethanol, or sodium ethoxide or sodium methoxide is necessary. In the case of B there is no condensation reaction since it has no α-hydrogens with respect to the carbonyl group.
Solution 5:
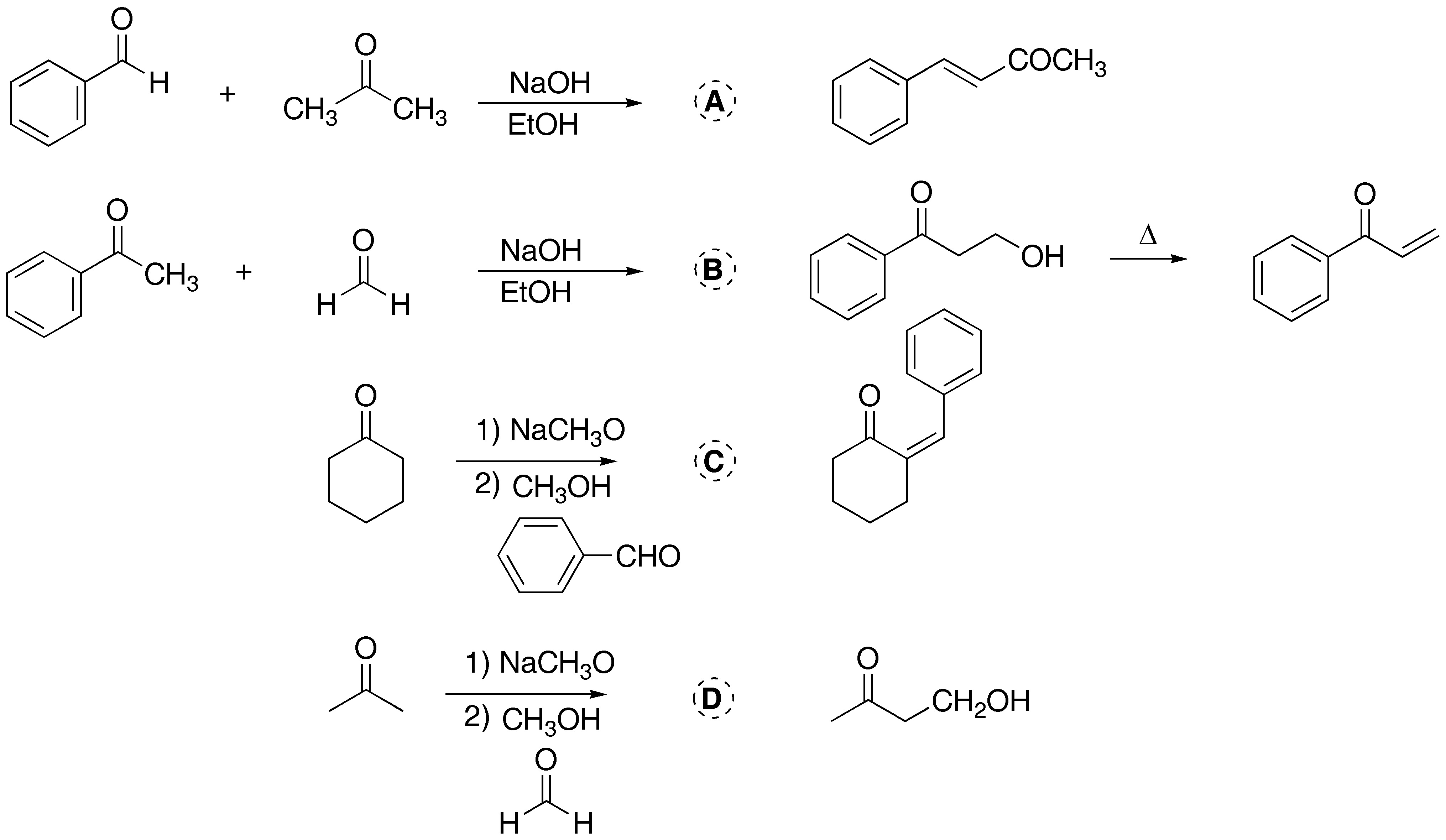
Solution 6:
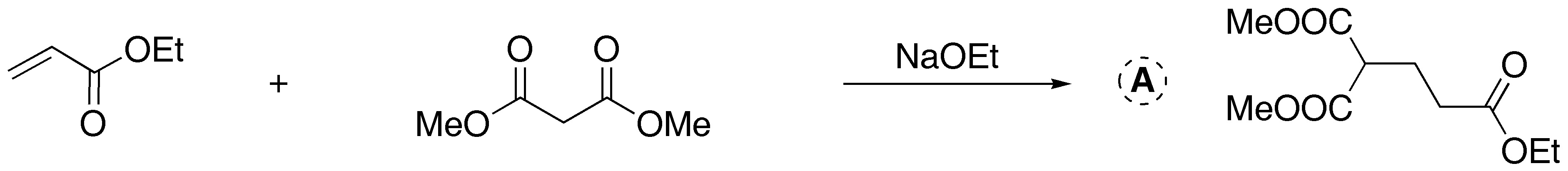
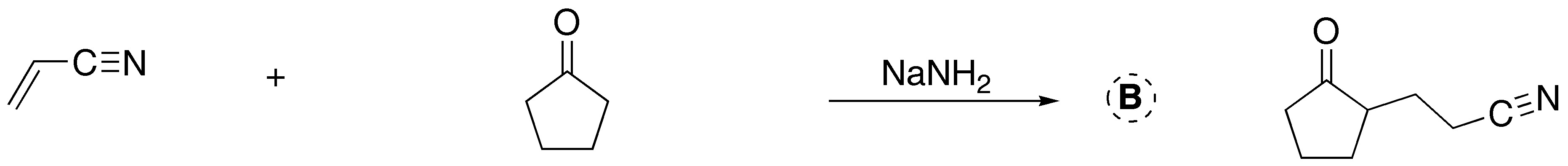
![]()
Solution 7:
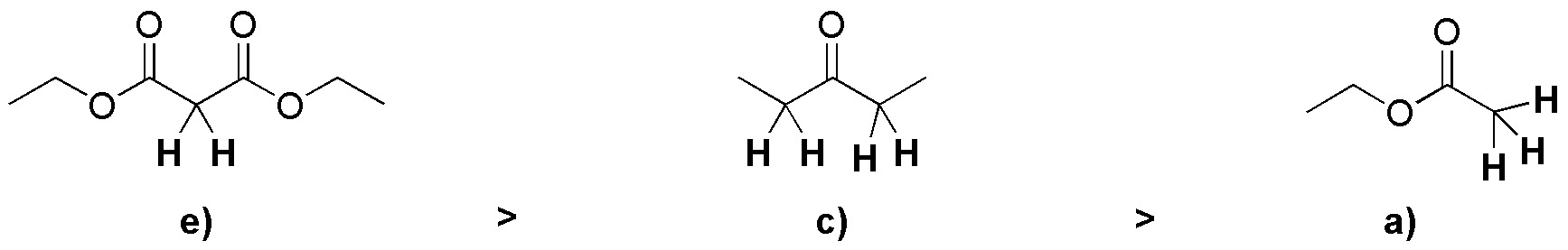
First we discard the compounds without α-hydrogens, these are b) and d). Then, we order the remaining compounds in decreasing order of acidity, resulting that first is the dicarbonyl ester, followed by the ketone, and finally the ester. The first two e) and c) being more acidic form enolates well with a base such as HO–. However the esters like a) being much less acidic, alkoxide type bases (EtO–) must be used to form the corresponding enolate.
Solution 8:
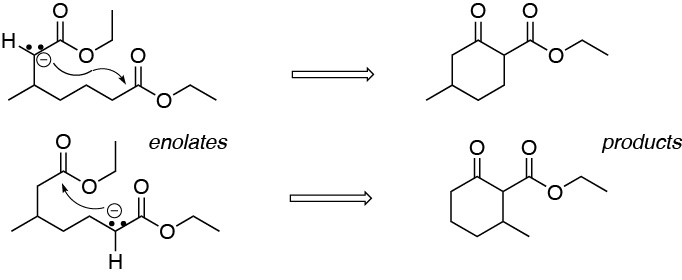
Since the starting dicarboxylic compound is not symmetrical, there is a possibility that two different enolates are formed. Therefore, the result of the reaction will be a mixture of two cyclization products arising from the attack of the negatively charged carbon on the carboxyl at the other end of the chain, generating in both cases a six-membered ring with the methyl substituent in a different position.
Solution 9:
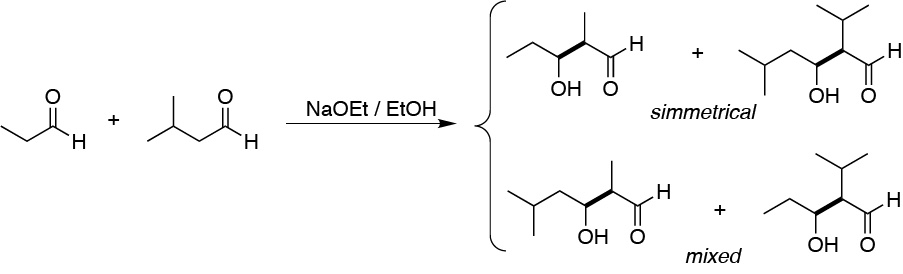
In basic medium, two enolate ions are formed which react respectively with the two starting aldehydes. The C-C bonds formed when the aldehydes react have been highlighted in bold.
Solution 10:
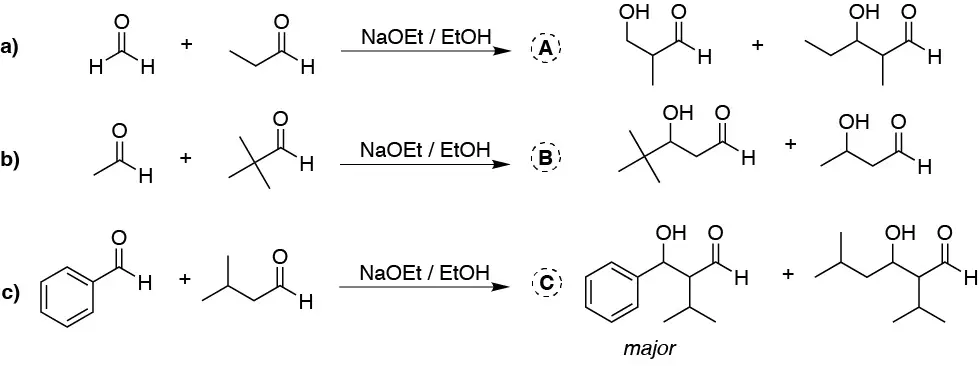
Unlike the previous problem, in all three reactions there is only one aldehyde with α-hydrogens. Both formaldehyde in a), pivalaldehyde in b), and benzaldehyde in c) have no hydrogen-α-hydrogens, so they are not enolizable. Therefore, two aldols are formed, one as a result of condensation between the two different starting aldehydes and the other from condensation of the enolizable aldehyde with another same starting aldehyde. In case c), as the phenyl group withdraws electrons from the carbonyl, it converts it into a more reactive carbonyl obtaining mostly the condensation product of the two reactants.
Solution 11:
In this cross aldol condensation, only one product is obtained mostly. This is because ethyl acetoacetate is a 1,3-dicarbonyl compound with the hydrogens located between the two carbonyl groups of the α-carbon more acidic than those of cyclohexanone. Therefore, it converts to the enolate much more easily than cyclohexanone which has only one carbonyl carbon, giving the product shown in the figure.
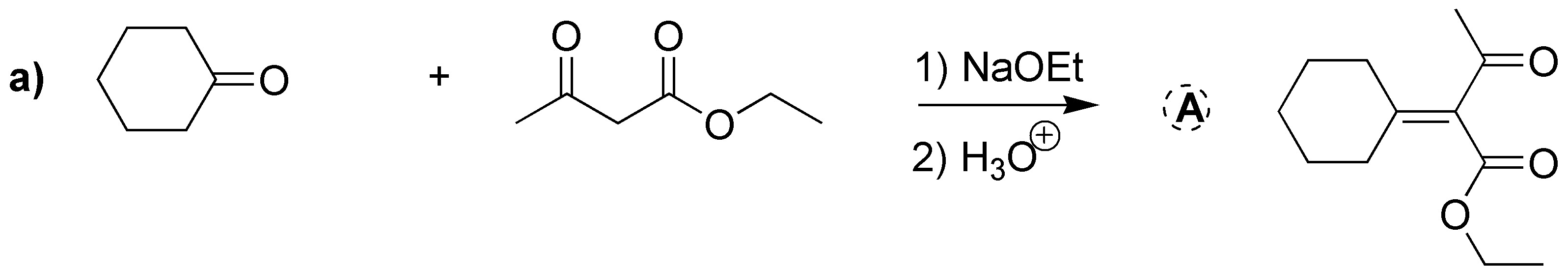
Solution 12:
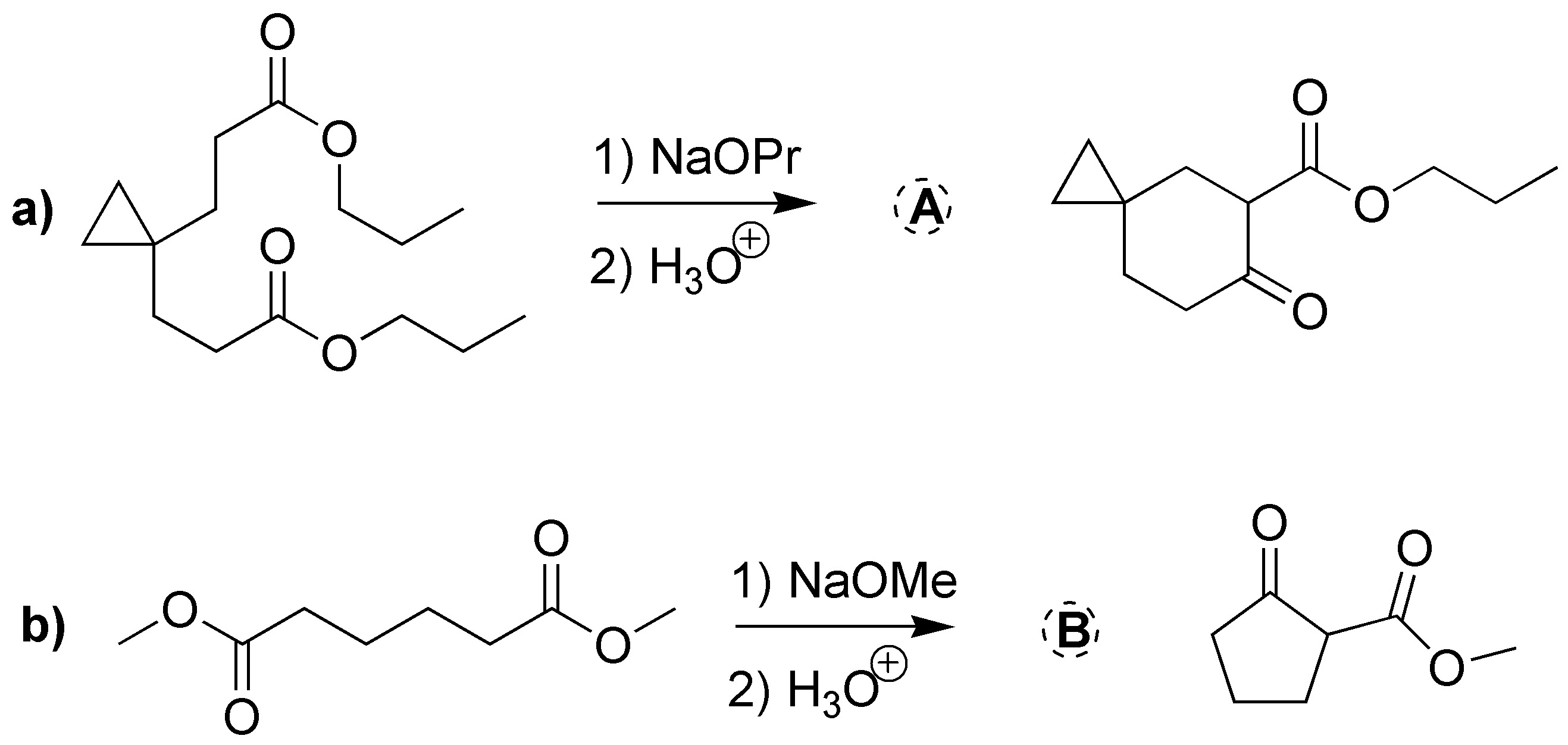
Both transformations are intramolecular Claisen reactions, in this particular case called Dieckmann condensation. The two esters used are symmetric, so a single enolate is formed which attacks the ester carbon at the end of the chain. Since the diester in reaction a) has seven carbon atoms the resulting cycle is six-membered, whereas in case b) there are six atoms in the chain supporting the two -COOCH3 groups, giving rise to a five-membered cycle.
Solution 13:
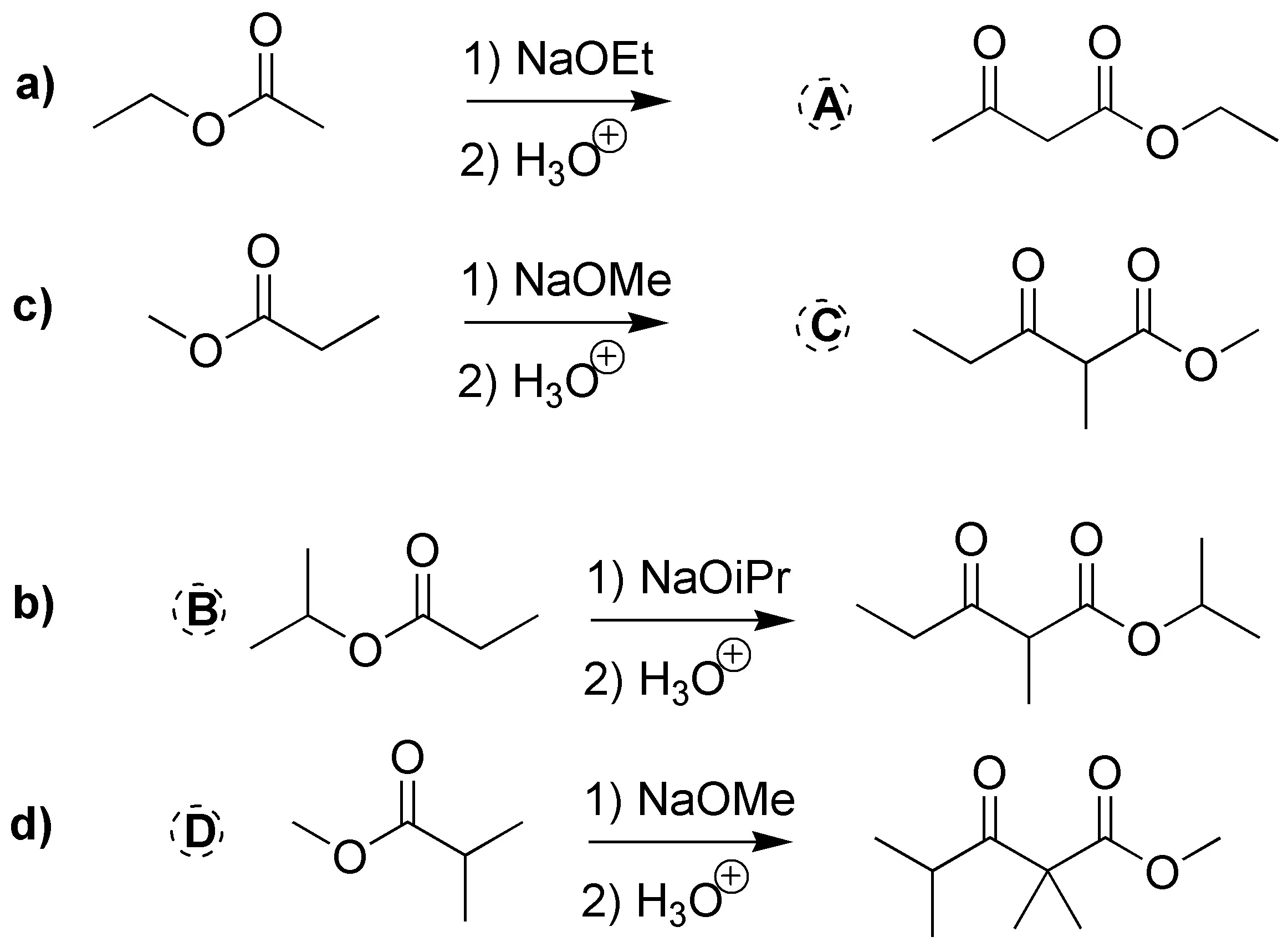
In this case four Claisen-type condensation reactions are involved. In all cases (a-d) we start from a single ester, so two moles of starting product must be consumed. One mole is spent in the production of the enolate and on the second mole the attack of the nucleophilic carbon of the enolate takes place.
From esters a and c the formation of compounds A and C indicated in the figure is deduced, while in transformations d and b the structure of the starting esters can be deduced by determining the nature of R1 and R2.
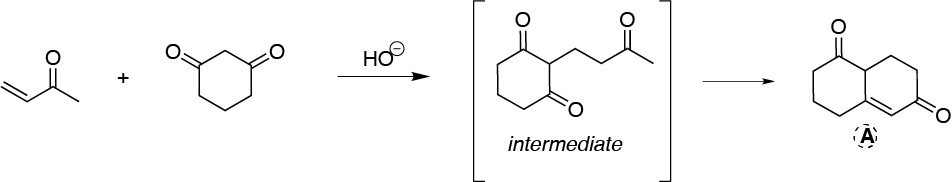
Solution 14:
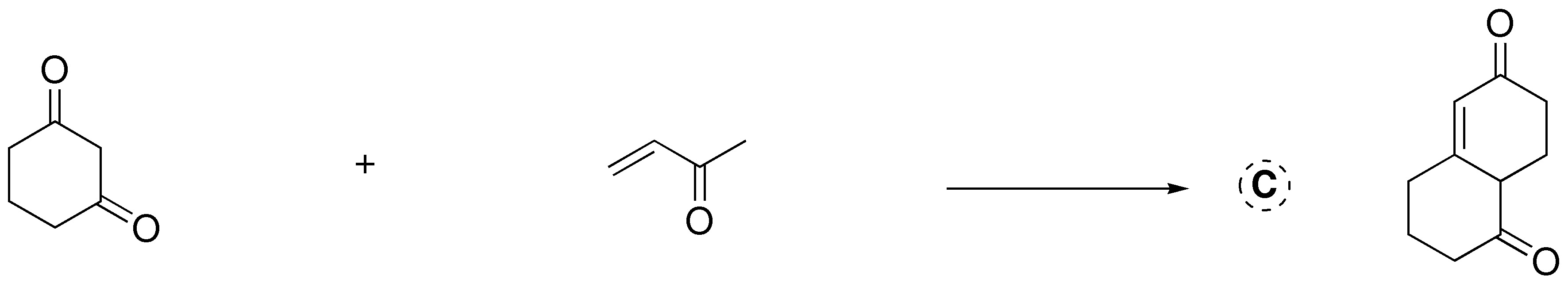
This is a Robinson ring formation reaction. First, the carbanion of cyclohexane-1,3-dione is formed on the carbon between the two carbonyl groups, since it is the most acidic (1,3-dicarbonyl compound) and is favored with respect to the α-carbon of but-3-en-2-one. This carbanion attacks the terminal carbon of the C=C double bond to give the Michael intermediate shown in the figure. Subsequently, this intermediate undergoes another loss of a proton at the methyl carbon-α to give an intramolecular aldol condensation, as it is favored to generate 6-membered rings.
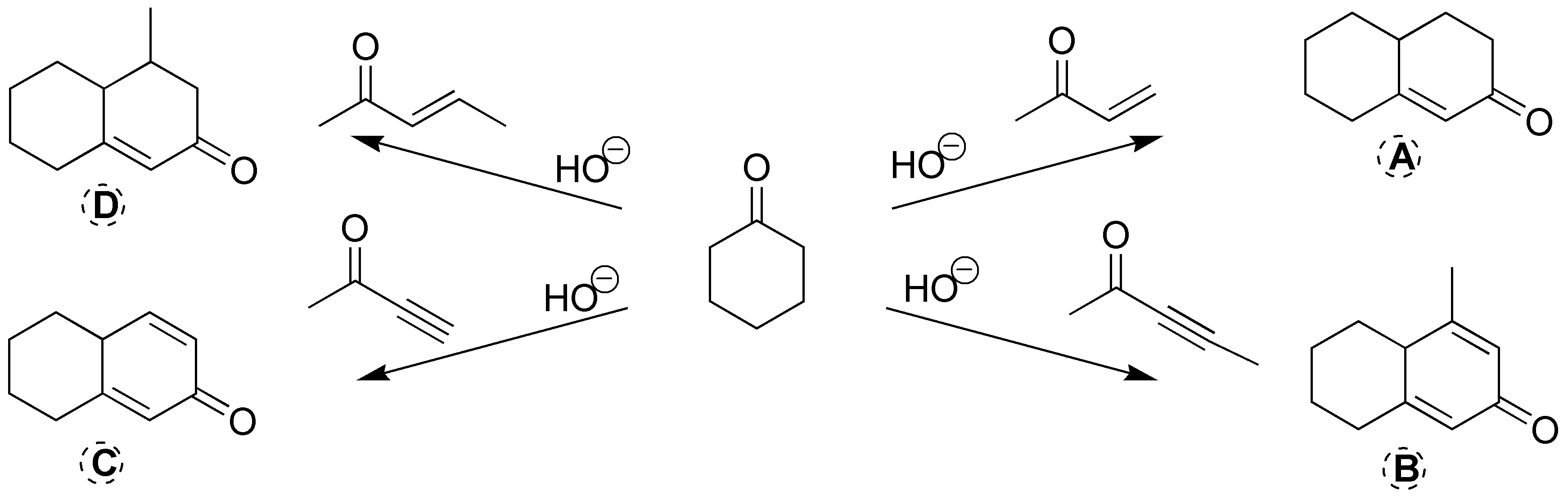
Solution 15:
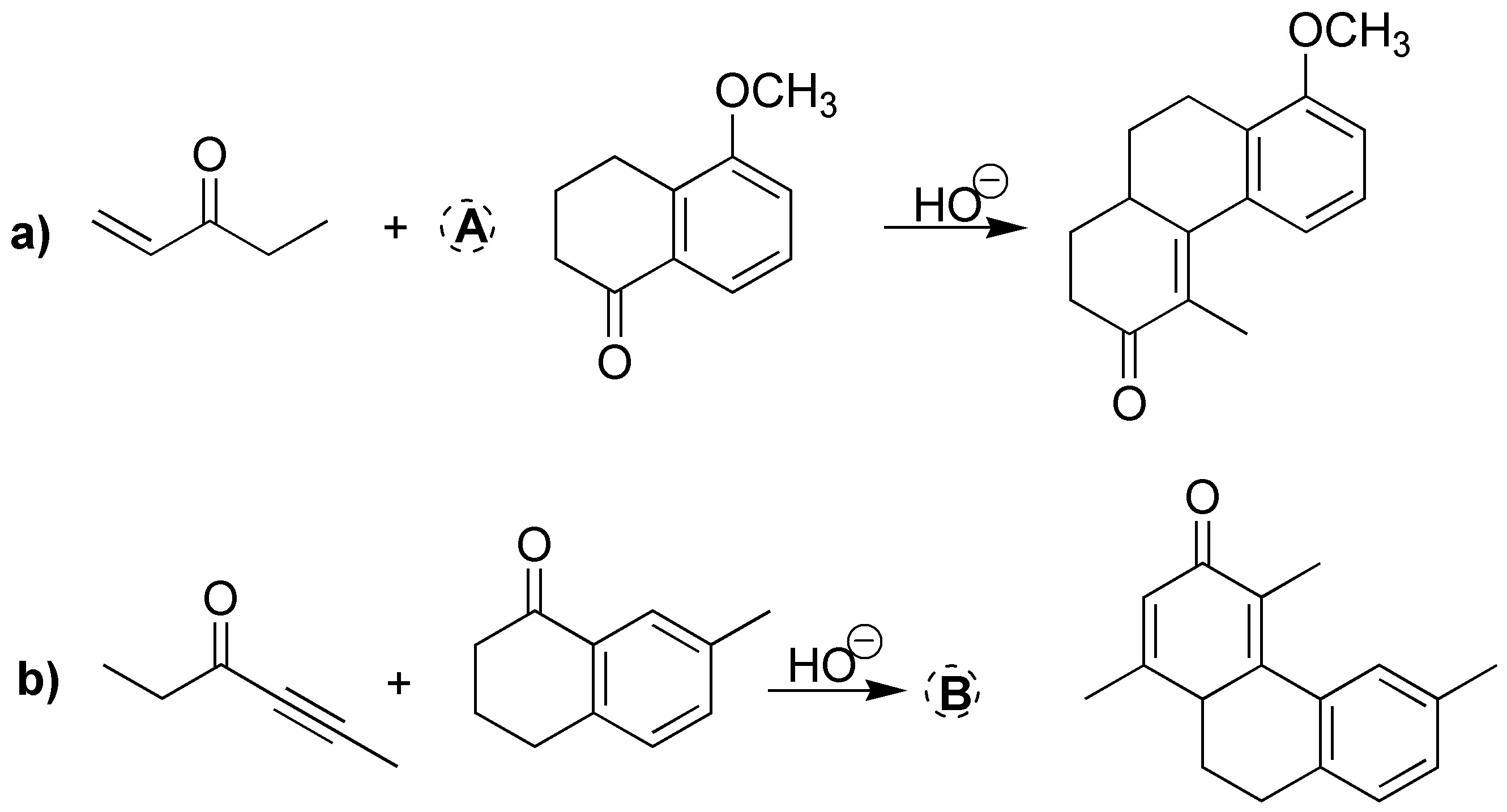
All four are Robinson ring-forming reactions. In basic medium, the reactants shown in the scheme lose the proton located on the carbon-α cyclohexanone. This carbanion is added to the carbon-carbon double and triple bonds of the other reactant indicated in the scheme. Subsequently, the aldol condensation and dehydration step takes place resulting in a double bond.
Solution 16:
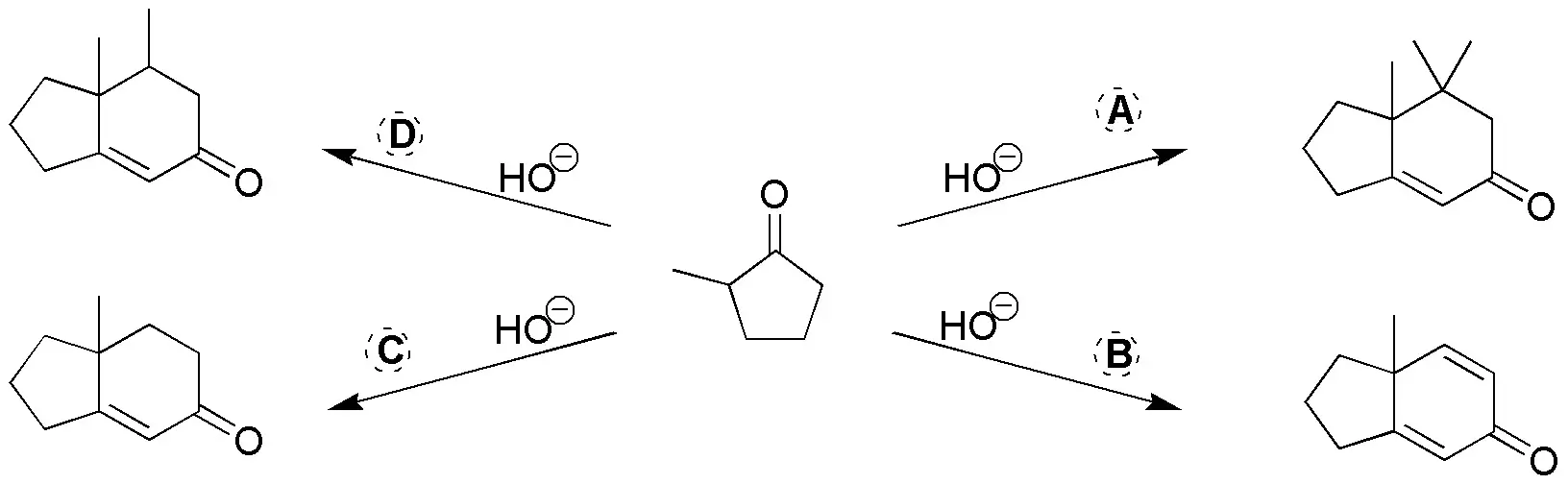
These are Robinson ring-forming reactions. First we take as reference the 2-methylcyclopentanone in the final products. Subsequently, we locate those new C-C bonds that are generated in the corresponding reactions (indicated in the following figure). In this way, the binding points between the starting product (2-methylcyclopentanone) and the reagents (A-D) that have been used are visualized.
One of these points of attachment is a double bond coming from the carbonyl group of the starting product and the carbon in position a of the reagent used (A-D).
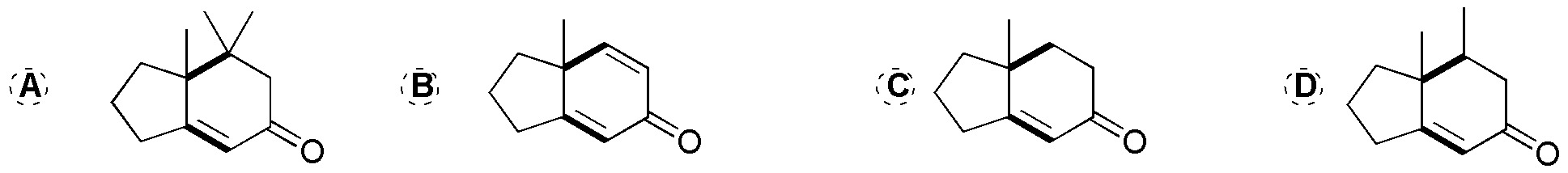
The other bonding point corresponds to the attack (Michael addition on the unsaturation of the reagent). If this unsaturation is a double bond in the final product, a single C-C bond is obtained, whereas if in the reagent we have a C≡C triple bond (case B), a C=C double bond is generated in the product. For clarity the fragment of the reagent that is incorporated into the final product is highlighted in bold in the following figure.
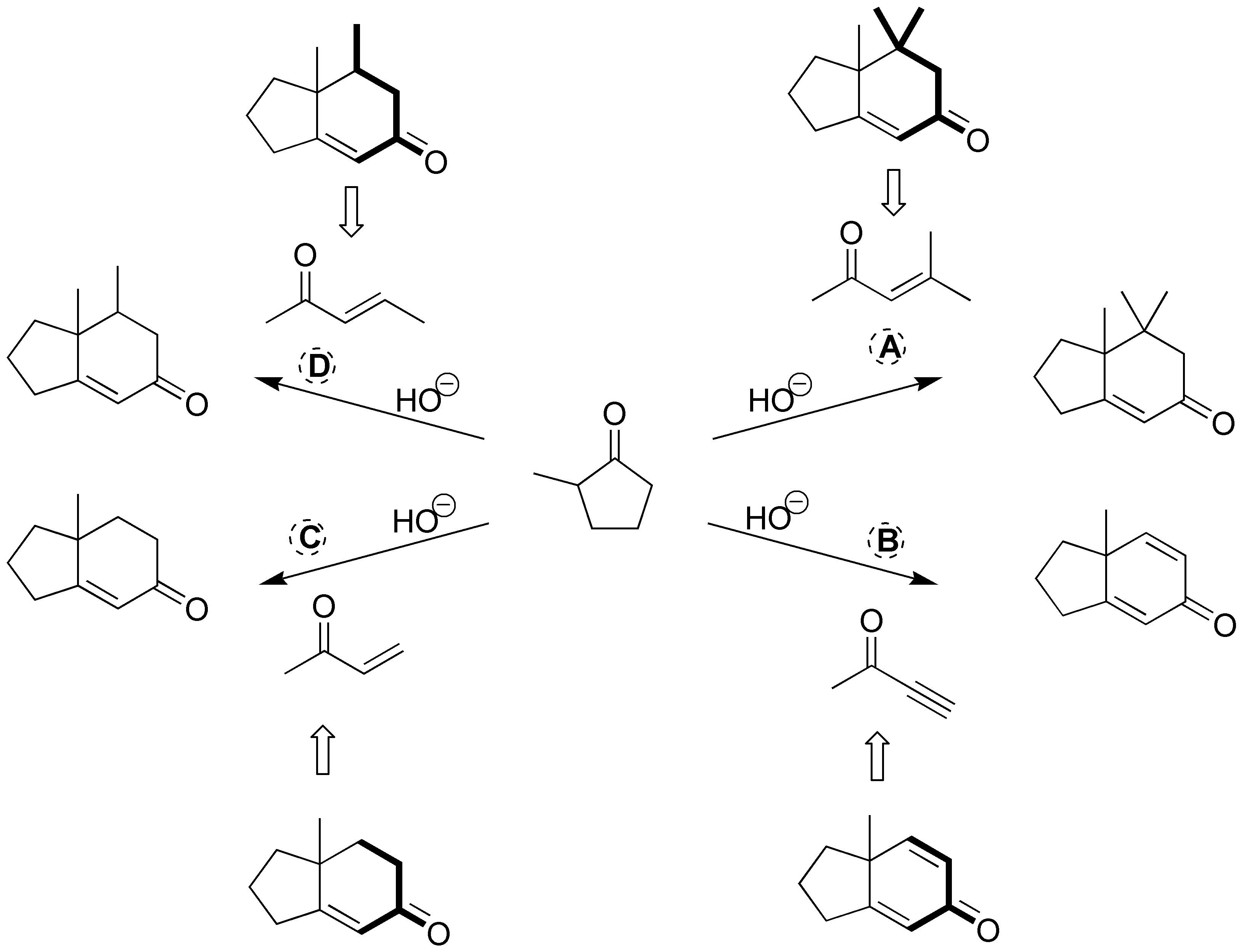
Solution 17:
Compounds b), d) and i) do not have α-hydrogens.
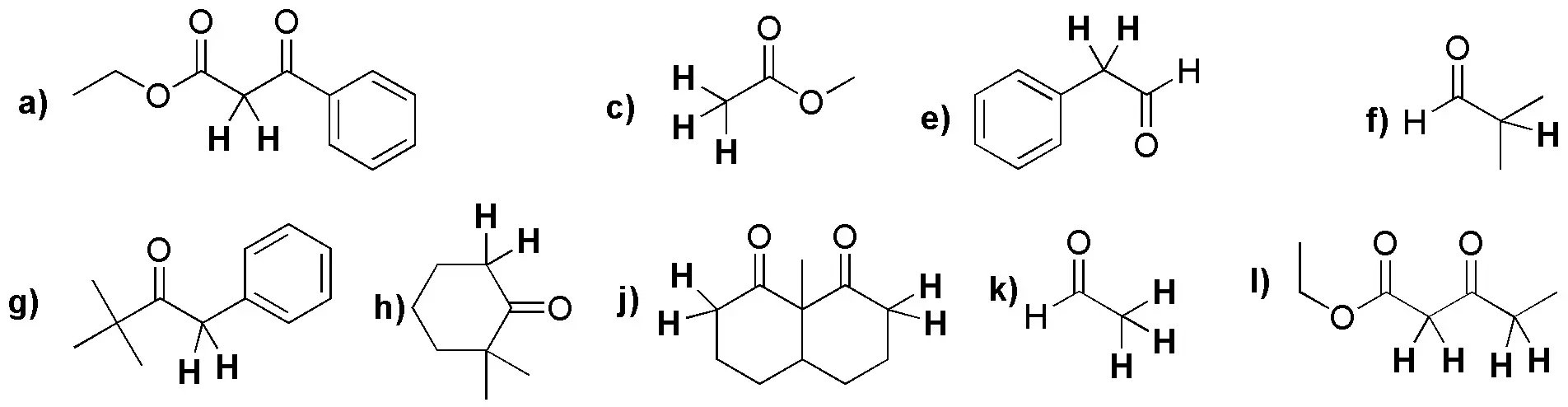
Solution 18:
Solution 19:
This is an intramolecular Claisen condensation (Dieckmann cyclization) in which the enolate of the lower ester will attack the upper ester forming the cyclopentanone ring:
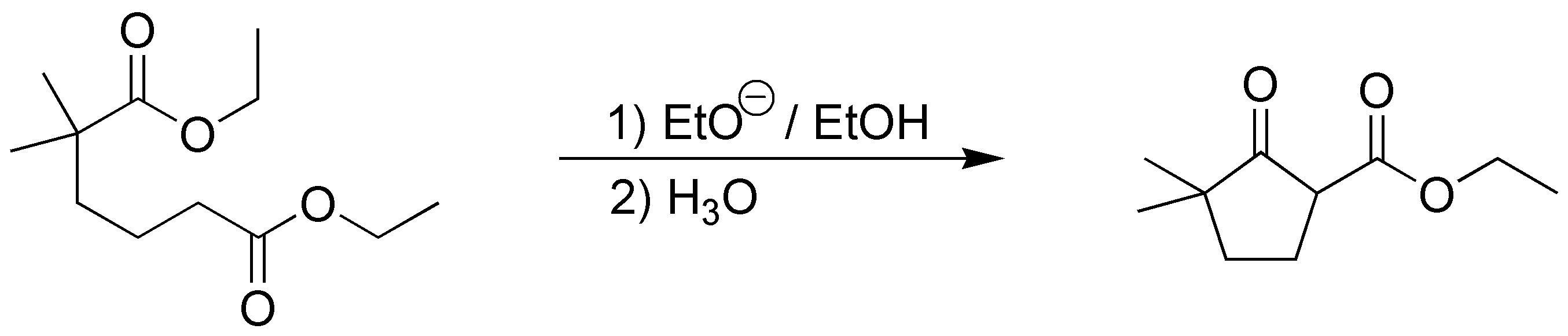
Solution 20:
Since there is a possibility of enolate formation of the two aldehydes, there will be 4 possibilities of aldol condensation: two symmetric and two cross:
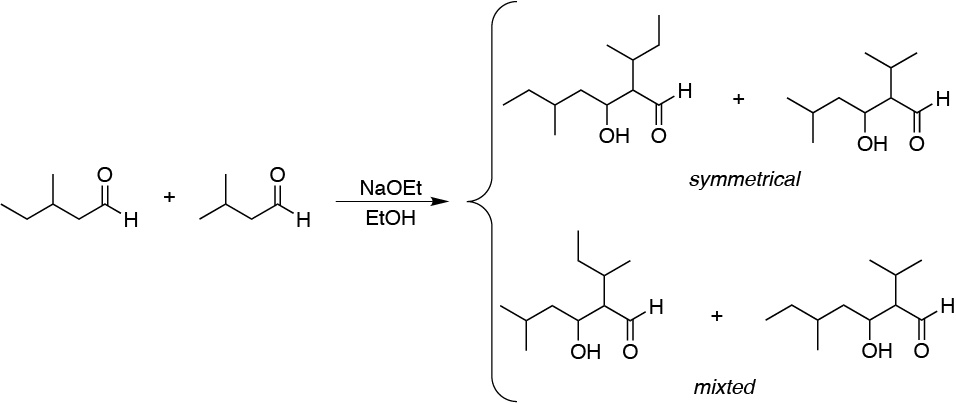
Solution 21:
This is a cross aldol condensation. Since the benzaldehyde has no hydrogen-α-hydrogens the enolate of the cyclohexanone will be formed which will attack the benzaldehyde giving the corresponding aldol.
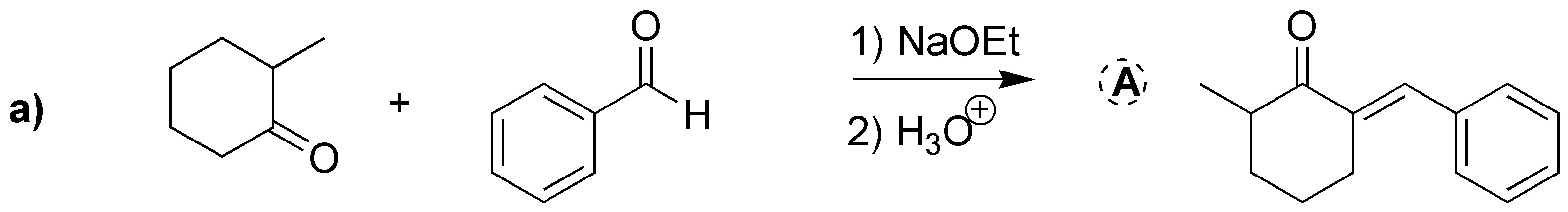
Solution 22:
These are two intramolecular Claisen condensations (Dieckmann cyclization):
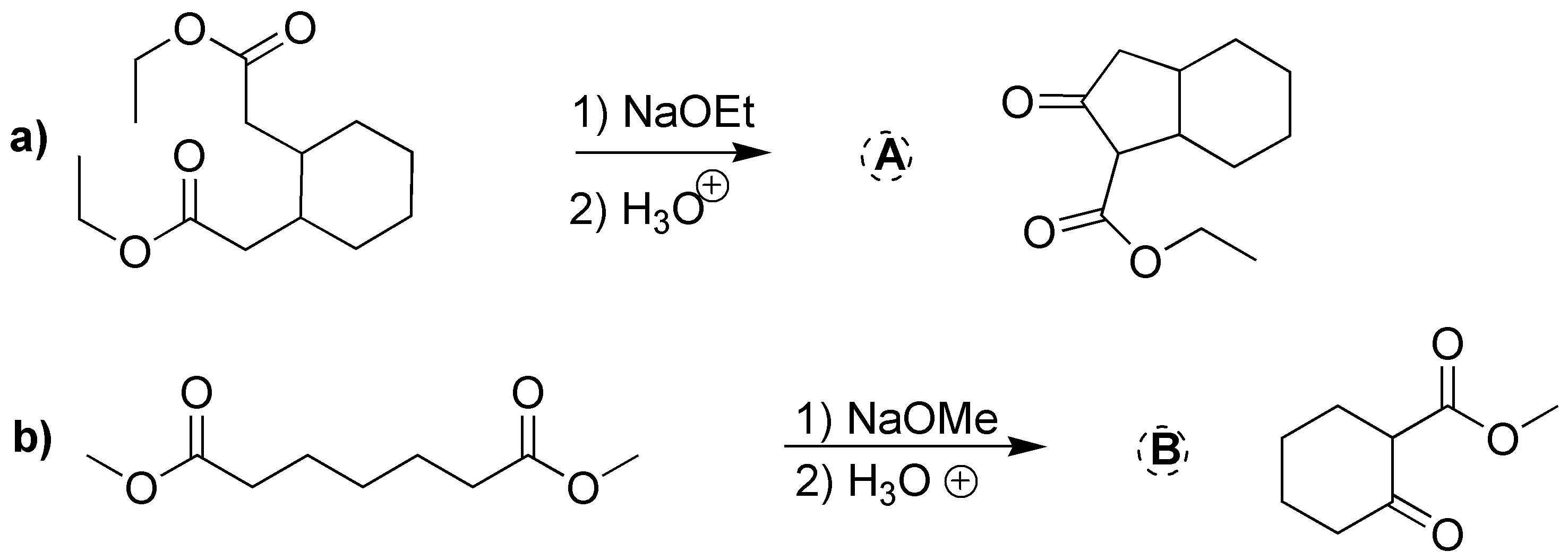
Solution 23:
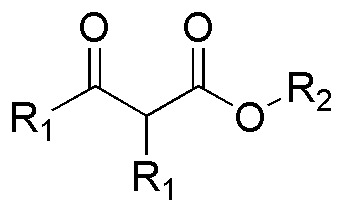
(a) and (b) correspond to two ketoesters, so they will proceed via two Claisen condensations: a) from isopropyl 2-methylpropanoate and b) from methyl propanoate. c) and d) are also two Claisen condensations that should give two ketoesters: c) ethyl 2,2,4-trimethyl-3-oxo-pentanoate and d) propyl acetylacetate (propyl 3-oxo-butanoate).
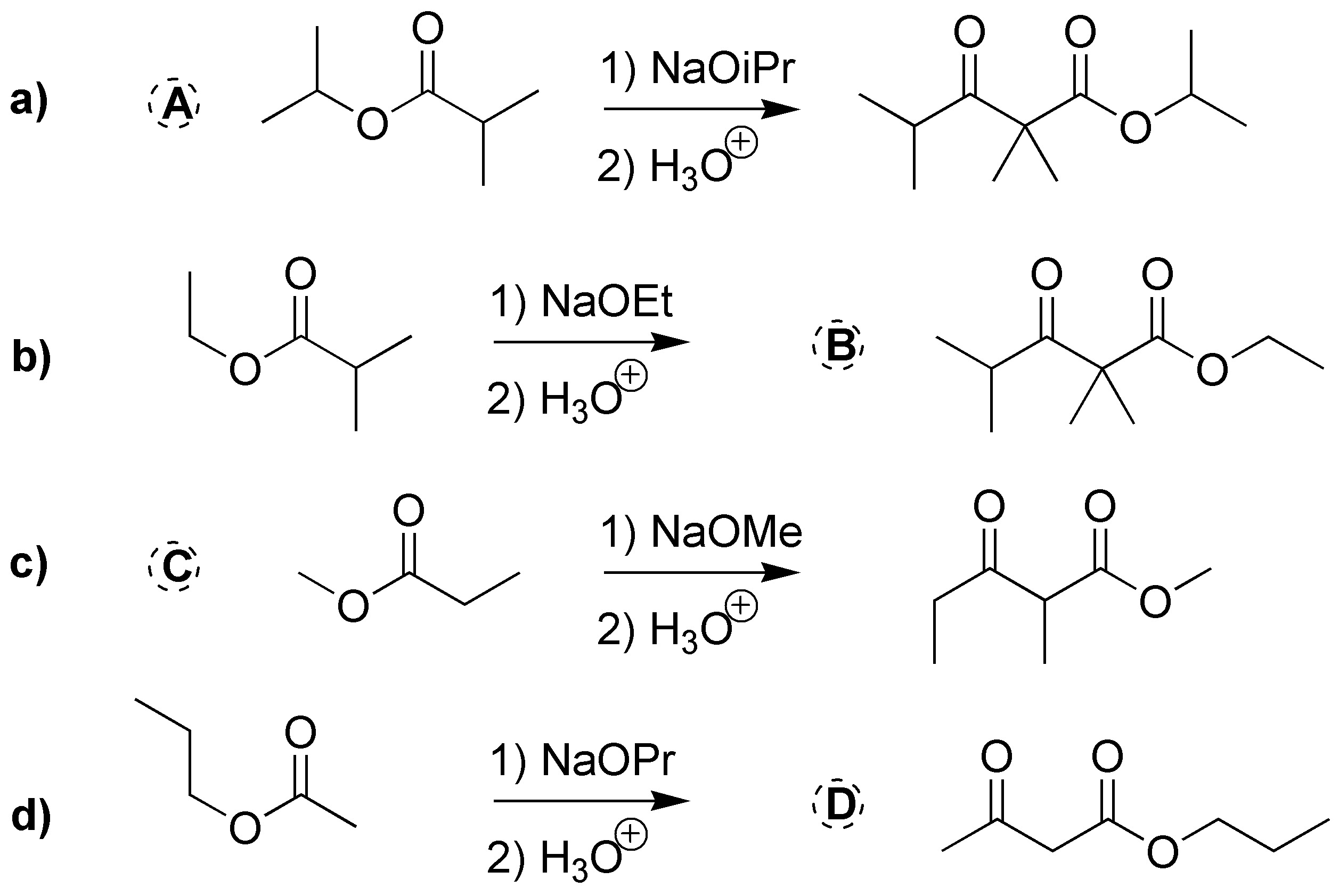
Solution 24:
We will be dealing with 4 Robinson ring-forming reactions, so the carbonyl compounds required are given in the following scheme: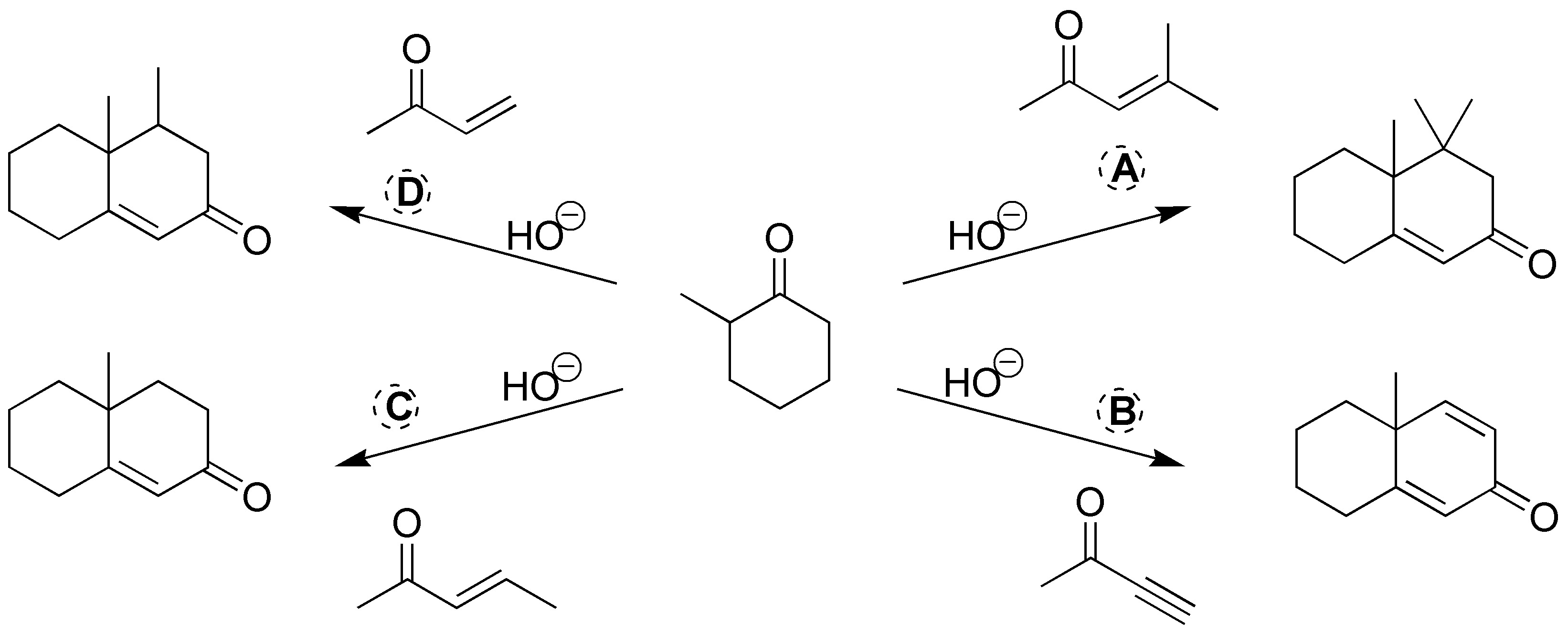
Solution 25:
We will be dealing with 4 Robinson ring formation reactions, so the compounds to be formed will be:
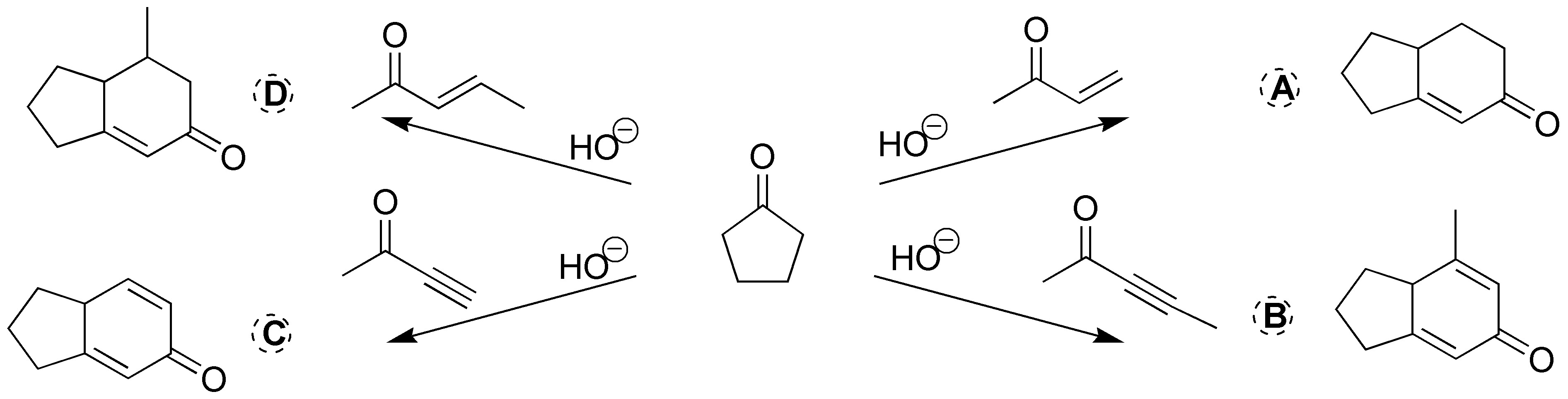
Solution 26:
We will be dealing with one Robinson ring formation reaction. The first step will be the result of the formation of the enolate and Michael type addition of this on the unsaturated carbonyl compound:
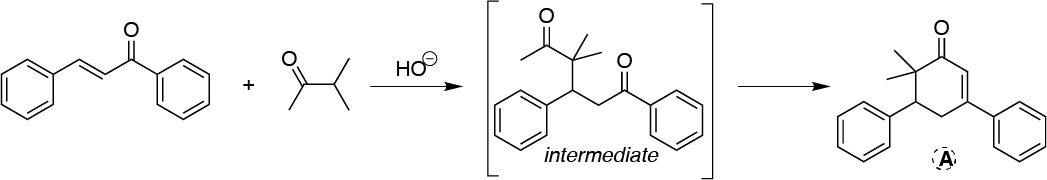
Solution 27:
(a) Corresponds to a Dieckmann cyclization. The enolate of the ester attacks the other ester giving a cyclic ketoester; b) it is a crossed aldol condensation; c) it is a Michael-type addition of a methylene active compound to an unsaturated carbonyl; d) it is an intramolecular aldol condensation; e) it is a Robinson ring formation reaction and f) it is a crossed Claisen condensation. The results of these are given in the following scheme:
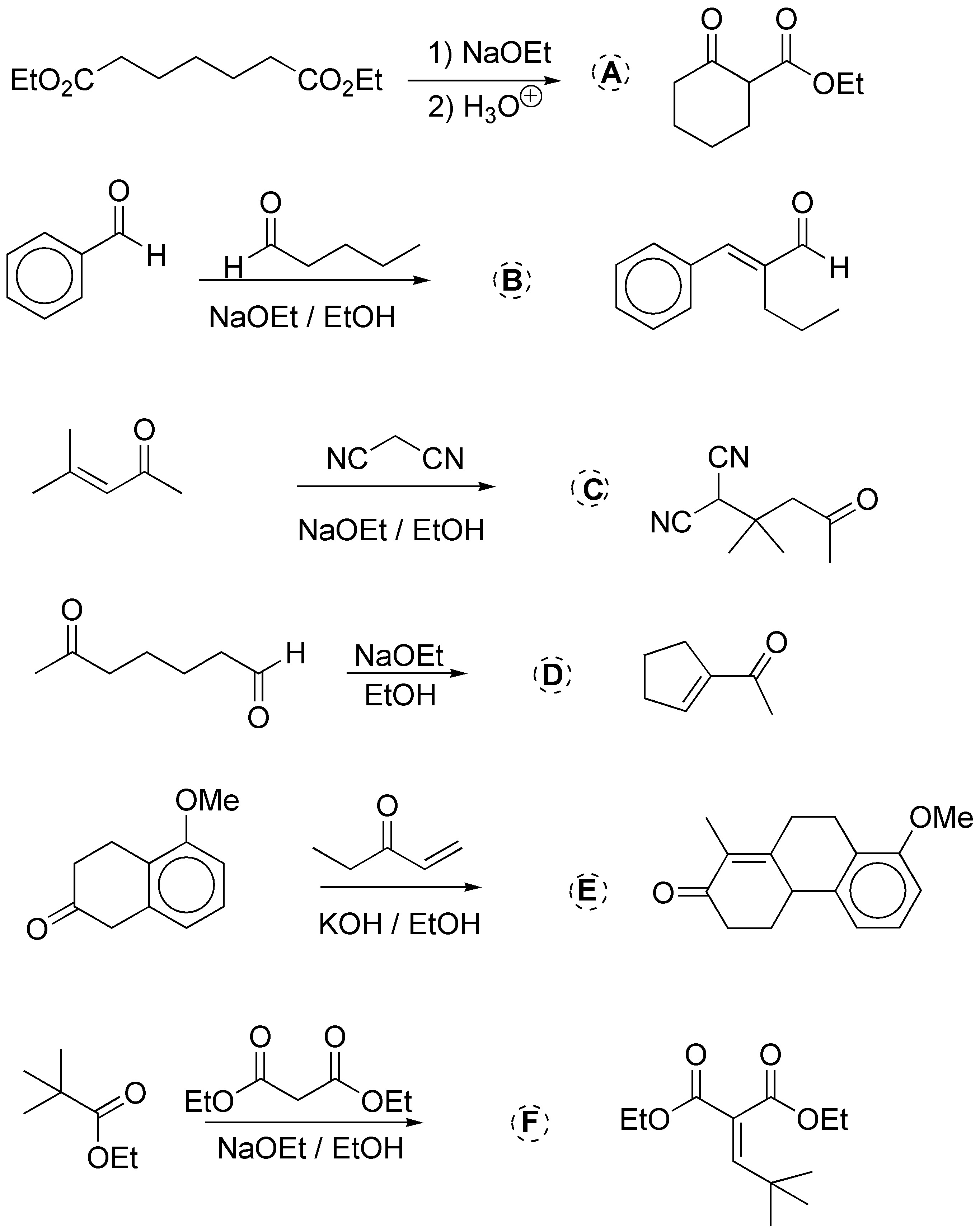
Solution 28:
Corresponds to two Robinson ring formation reactions. In the first one, we must find the carbonyl compound for which we must indicate that part of the final structure corresponds to the unsaturated carbonyl compound. In the second, we must indicate the intermediate that is formed by Michael-type addition of the enolate of the ketone on the triple bond and then perform an aldol condensation.