Written by J.A Dobado | Last Updated on April 22, 2024
What is vitamin A?
Vitamin A is a group of unsaturated nutritional organic compounds that includes: retinol, retinal, retinoic acid and various provitamin A carotenoids. Of the latter the most notable is β-carotene (beta-carotene).
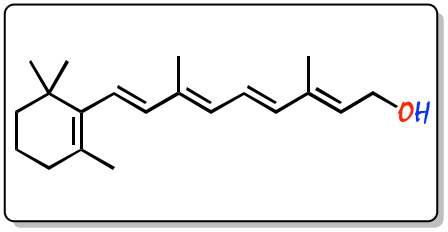
The IUPAC systematic name for retinol is (2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraen-1-ol.
Chemical structure
In 1929-1931, Paul Karrer (Nobel Prize in Chemistry in 1937) determined the structure of vitamin A for the first time. All forms of vitamin A (retinol, retinal and retinoic acid) have, in the polyene chain, four of the five double bonds that can give rise to different geometric isomers (cis or trans configuration). However, the cis isomers are less stable and can be easily converted to the all-trans configuration. On the other hand, some cis isomers occur naturally and perform essential functions in the body. For example, the 11-cis-retinal isomer is the chromophore of rhodopsin, the vertebrate photoreceptor molecule.
Rhodopsin consists of the 11-cis-retinal molecule covalently linked through a Schiff base to the opsin protein. Thus, the vision process is based on light-induced isomerization of the 11-cis to trans chromophore resulting in a conformational change and activation of the photoreceptor molecule.
 |
| 3D Structure |
Industrial production
Retinol can be obtained by three synthetic routes, which are used industrially and use the β-ionone molecule as a starting point.
Arens-van Dorp synthesis of intermediate C21
Arens and Van Dorp (in Organon International) performed in 1946-1947 a linear multistage synthesis from β-ionone in which they obtained an acid. From that compound, in the following stages they systematically elongate the unsaturated carbon side chain by alkylation and interconversion of functional groups. The esters are converted to acids by saponification and finally to the corresponding ketone by the action of methyl lithium (MeLi). In this way the intermediate C21 is obtained.
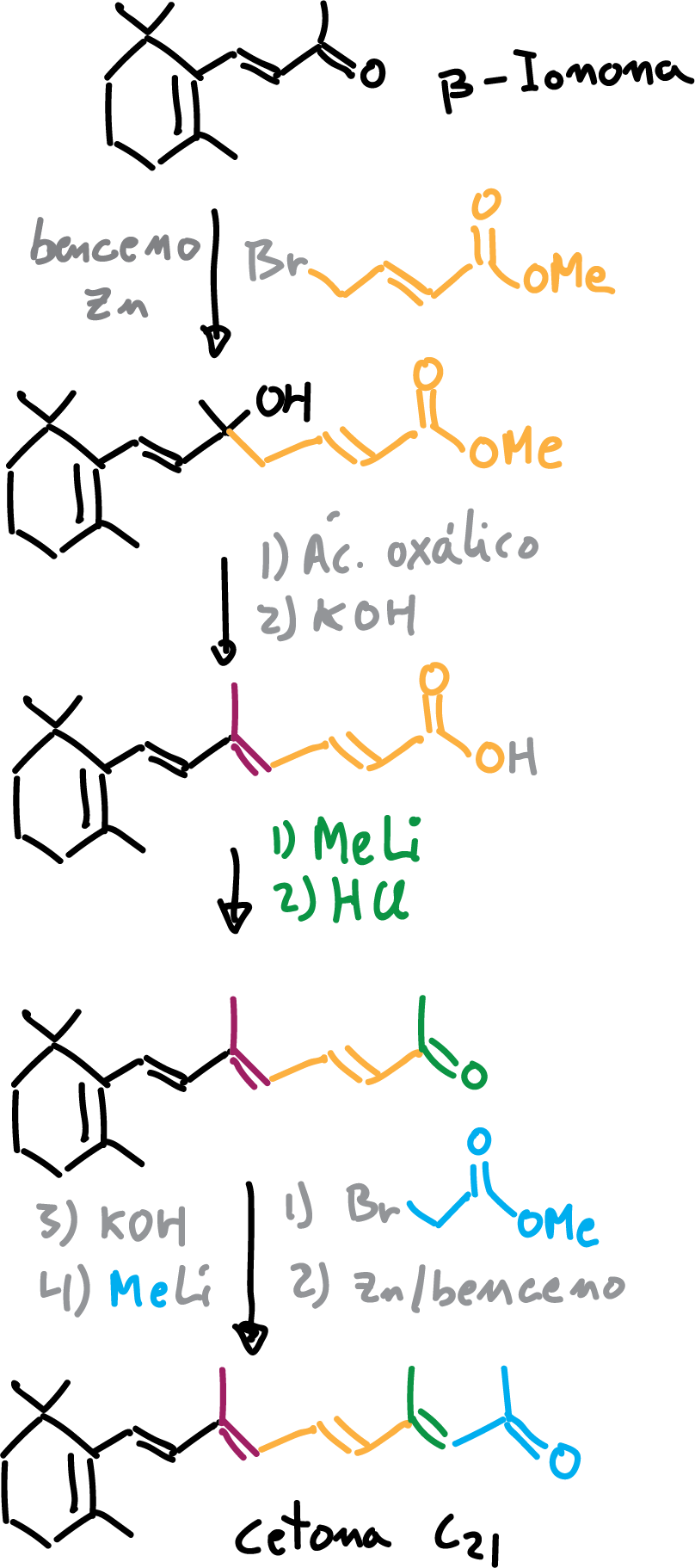
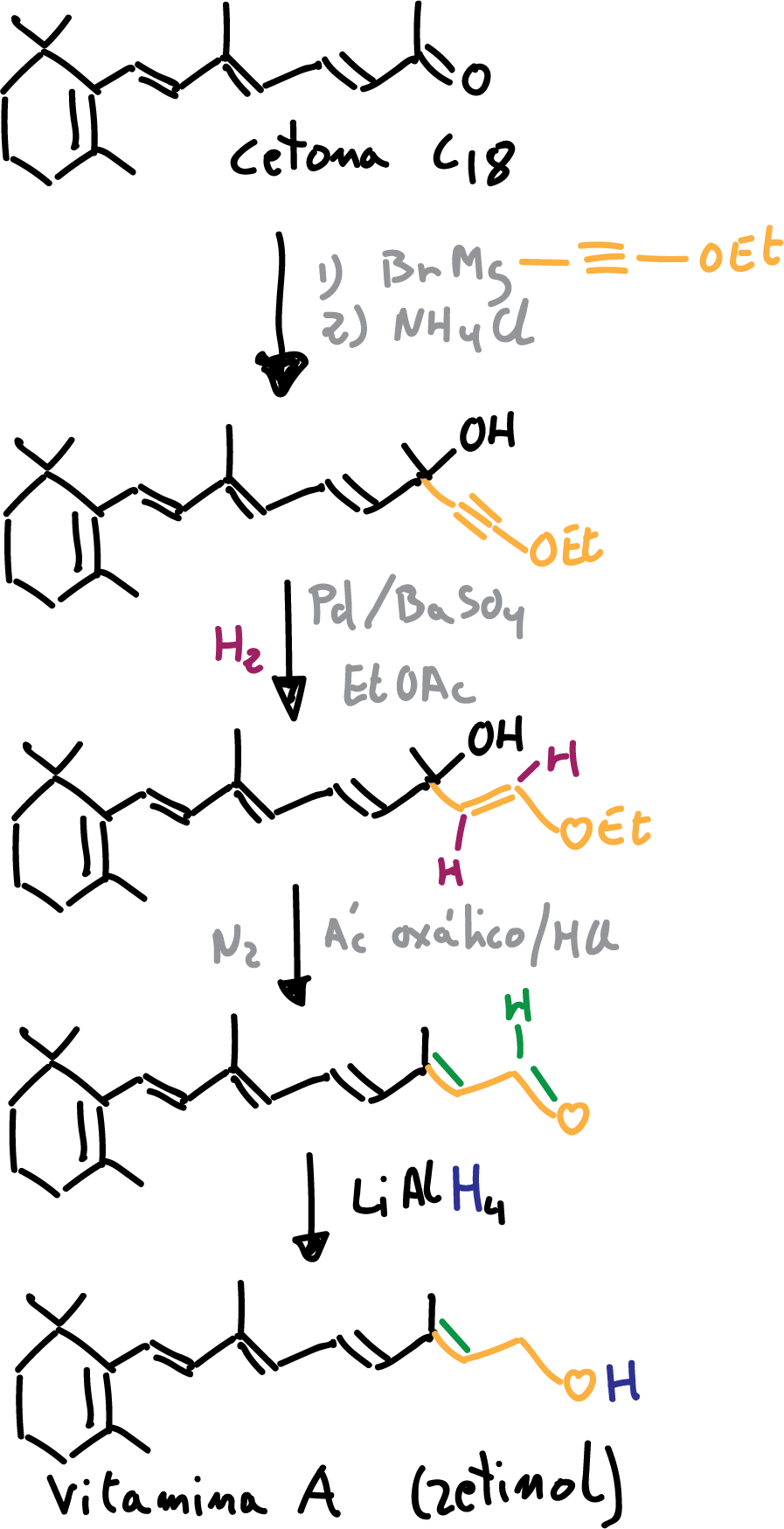
Functions
George Wald received the 1967 Nobel Prize for his work with retinal pigments, which contributed to the understanding of the role of vitamin A in vision. One of the first signs of vitamin A deficiency is night blindness followed by decreased visual acuity.
Vitamin A and β-carotene are antioxidants, help protect against cancer and improve resistance to certain diseases. Also, they help form and maintain a healthy function of eyes, hair, teeth, gums and mucous membranes. In addition, vitamin A is involved in fat metabolism and white blood cell production. It is a fat-soluble vitamin that occurs in two forms: retinol, which is found in animal tissues, and β-carotene, which is found in plants. β-carotene is sometimes called a provitamin because the body must break it down into vitamin A before it acts as a vitamin.
Food sources
Whole milk, butter, offal, carrots, sweet potatoes, cabbage, pumpkin, spinach, arugula, red peppers, dark green vegetables, liver, cheese, fish liver oil, egg yolks and apricots.