What is Zincke nitration?
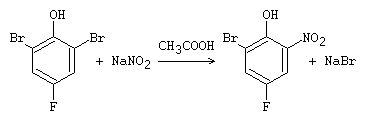
The Zincke nitration is a chemical reaction that involves replacing ortho– or para-bromine or iodine atoms in phenols with a nitro group upon treatment with nitrous acid, HNO2, or a nitrite in acetic acid. It is worth noting that this reaction cannot be used with fluorine or chlorine atoms, as shown in the accompanying figure.
References
Zincke, T. (1900), Ueber die Einwirkung von salpetriger Säure auf Brom- und Chlorderivate von Phenolen. [On the effect of nitrous acid on bromine and chlorine derivatives of phenols.] J. Prakt. Chem., 61: 561-567. https://doi.org/10.1002/prac.19000610145
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.