What is Bamberger rearrangement?
The Bamberger rearrangement, also known as the Bamberger reaction, involves an intermolecular rearrangement of N-phenylhydroxylamines in acidic aqueous solutions, leading to the production of the corresponding 4-aminophenols.
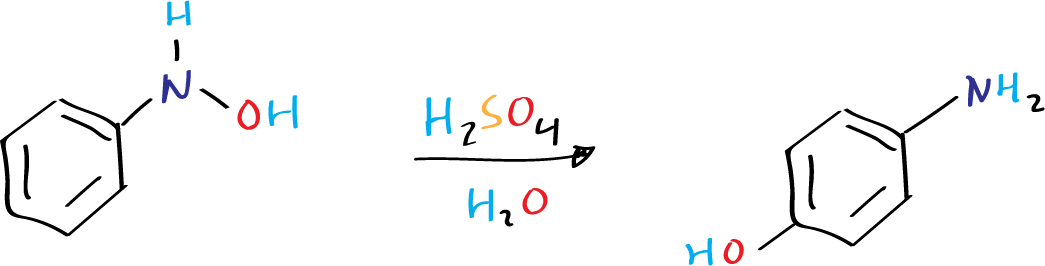
The reaction was first described by Bamberger in 1894 and is thought to occur through a nucleophilic attack of a hydrosulfate ion on the anilenium ion, which is formed via heterolytic N-O bond cleavage of O-protonated N-phenylhydroxylamine.
Mechanistic evidence supports the heterolytic N-O bond cleavage, and incorporation of alkoxy, halogen, and phenoxy groups into the aromatic ring has been observed when the rearrangement is carried out in nucleophilic solvents such as alcohols, hydrogen halides, and phenols.
References
- Bamberger, E. (1894), Ueber die Reduction der Nitroverbindungen. [On the reduction of nitro compounds.] Ber. Dtsch. Chem. Ges., 27: 1347-1350. https://doi.org/10.1002/cber.18940270229
- Bamberger, E. (1894), Ueber das Phenylhydroxylamin. [On phenylhydroxylamine.] Ber. Dtsch. Chem. Ges., 27: 1548-1557. https://doi.org/10.1002/cber.18940270276