What is Wessely-Moser rearrangement?
The Wessely-Moser rearrangement is a chemical reaction that is specifically performed on flavones and flavanones containing a 5-hydroxyl group.
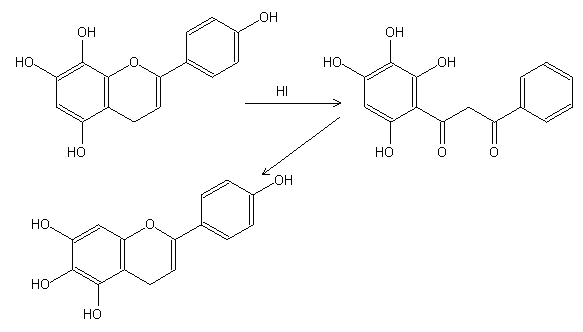
During the Wessely-Moser rearrangement, the heterocyclic ring undergoes fission and the resulting intermediate diaroylmethanes subsequently reclose in the opposite direction.
References
Wessely, F., Moser, G.H. Synthese und Konstitution des Skutellareins. [Synthesis and constitution of the scutellar stone.] Monatshefte für Chemie 56, 97–105 (1930)
DOI: 10.1007/BF02716040
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.