What is Tiffeneau-Demjanov rearrangement?
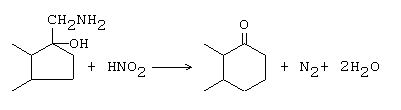
The Tiffeneau-Demjanov rearrangement refers to a chemical reaction that involves the expansion of the ring structure of β-amino alcohols upon diazotization with nitrous acid, resulting in the formation of carbonyl compounds. In addition to this, cyclic alcohols can also yield ring expanded or contracted products through this reaction.
References
- M. Tiffeneau, P. Weill, B. Tchoubar, Isomérisation del’oxyde de méthylène cyclohexane en hexahydrobenzaldéhyde et désamination de l’aminoalcool correspondant en cycloheptanone
[Isomerization of cyclohexane methylene oxide to hexahydrobenzaldehyde and deamination of the corresponding aminoalcohol to cycloheptanone] Comptes rendus de l’Académie des sciences 205, 54-56 (1937)
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.