What is Lossen rearrangement?
The Lossen rearrangement is a chemical reaction that involves the conversion of a hydroxamic acid into an isocyanate, which can be achieved through the use of its O-acyl, sulfonyl, or phosphoryl derivative. In the presence of amines, ureas are formed. Furthermore, when water is present, amines containing one less carbon than the starting material can be obtained.
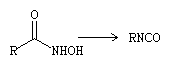
References
Lossen, W. (1872), Ueber Benzoylderivate des Hydroxylamins. [On benzoyl derivatives of hydroxylamine.] Justus Liebigs Ann. Chem., 161: 347-362. https://doi.org/10.1002/jlac.18721610219
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.