Objective
To perform the oxidation of an aldehyde to carboxylic acid, avoiding the use of transition metals (Cr, Mn, etc.), specifically the oxidation by Oxone®.
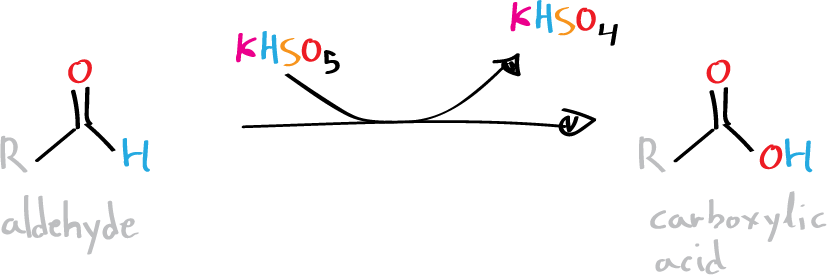
Background
Oxone® is the trade name of a triple salt with a molecular mass of 614 g/mol, formed by potassium peroxymonosulfate (KHSO5), potassium hydrogen sulfate (KHSO4), and sulfate potassium (K2SO4) in a 2:1:1 ratio, respectively. The strongly oxidizing character of this product is given by the presence of KHSO5, which can oxidize numerous compounds such as alkenes (transforming terminal alkenes into epoxides) or transforming aldehydes to yield carboxylic acid. Using Oxone®, oxidants such as transition metals (Cr and Mn) are avoided, and the procedure has a lower environmental impact.
Experimental procedure
To a 50 ml round-bottom flask, add 1.0 g of benzaldehyde, 7.25 g of Oxone®, and 25 ml of deionized water. Attach a water condenser (reflux) to the flask, and heat in a water bath at 60 ºC for 75 min with magnetic stirring.
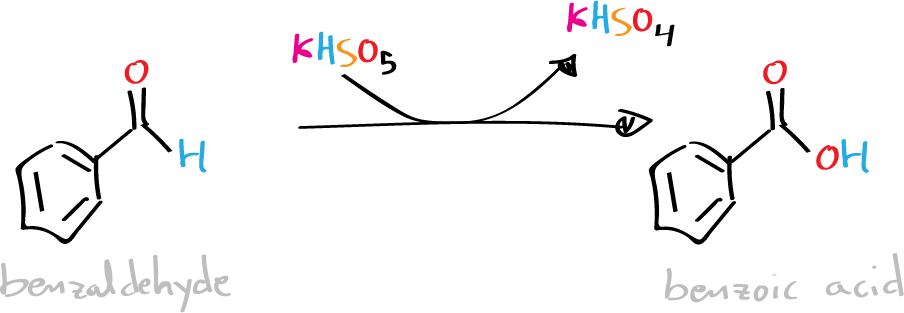
Afterward, cool the reaction in an ice bath for 15 min until a precipitate appears. Filter the solid under vacuum, and if necessary, drag the remaining solid residues in the flask with the minimum amount of water. Finally, flush the solid in a Büchner with 10 ml of cold water. Dry the solid by pressing it between two pieces of filter paper, weigh it, and determine the yield. Recrystallize the benzoic acid from water and determine the melting point (m.p.).
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Benzaldehyde | 106.12 | -26 | 178-179 | 1.044 |
| Oxone® | 614.78 | - | - | - |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Benzaldehyde |  |
| Oxone® |     |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Benzaldehyde | HUMNYLRZRPPJDN-UHFFFAOYSA-N |
| Oxone® | HJKYXKSLRZKNSI-UHFFFAOYSA-I |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.