Objective
To determine the density of a solid and a liquid and compare the values with their known densities.
Background
The density of a solid and liquid experiment is a classic laboratory experiment. The experiment aims to determine the density of a solid and a liquid and compare the values with their known densities.
The density of a substance is defined as its mass per unit volume:
ρ (g/L) = M / V
In this experiment, the density of a solid and a liquid will be determined and compared with their known densities.
This experiment provides students with hands-on experience in measuring mass and volume and applying the density formula, which is defined as the mass per unit volume of a substance. Also it helps to develop their laboratory skills, including measurement, data analysis, and report writing.
Materials and methods
- A balance
- A graduated cylinder or pipette
- A ruler
- A solid sample
- A liquid sample
Experimental procedure
- Prepare the apparatus: The apparatus for this experiment typically consists of a balance, a graduated cylinder or a pipette, a ruler, and a solid and a liquid sample.
- Measure the mass of the solid: Place the solid sample on the balance and record its mass.
- Determine the volume of the solid: The volume of the solid can be determined by several methods, such as displacement of water, immersion in a graduated cylinder, or measurement with a ruler.
- Calculate the density of the solid: The density of the solid can be calculated by dividing its mass by its volume.
- Repeat the procedure for the liquid: Measure the mass and volume of the liquid sample and calculate its density.
- Compare the calculated densities with the known densities: The calculated densities can be compared with the known densities of the solid and liquid, which can be found in reference materials or from previous experiments.
- Analyze the results and write a report: The results of the experiment should be analyzed and compared with the known densities. A report should be written, summarizing the procedure, results, and conclusions of the experiment.
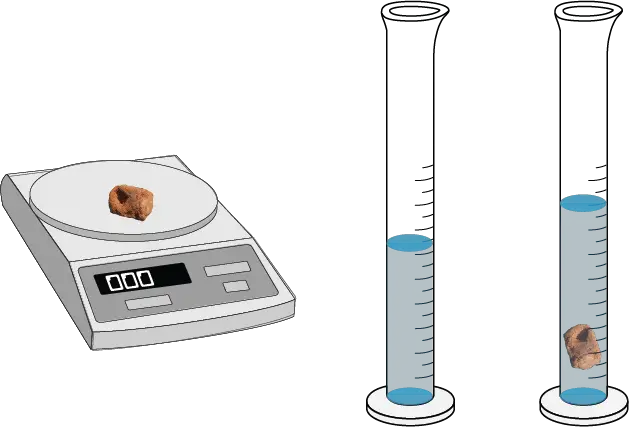
The experiment was performed as follows:
- The mass of the solid sample was measured on the balance and recorded.
- The volume of the solid sample was determined by displacement of water in a graduated cylinder or by measurement with a ruler.
- The density of the solid was calculated by dividing its mass by its volume, ρ (g/L) = M / V.
- The mass and volume of the liquid sample were measured and its density was calculated in the same manner as the solid.
- The calculated densities were compared with the known densities of the solid and liquid.
Lab report results
The mass of the solid sample was measured to be “insert mass“. The volume of the solid was determined to be “insert volume“. The density of the solid was calculated to be “insert density“.
The mass of the liquid sample was measured to be [insert mass]. The volume of the liquid was determined to be “insert volume“. The density of the liquid was calculated to be “insert density“.
The calculated densities of the solid and liquid were compared with the known densities and found to be “insert results“.
Sources of Error
Insert in the lab report sources of error, such as measurement error, calculation error, or contamination of the sample.
Future Work
Insert in the lab report suggestions for future work, such as repeating the experiment with a different solid or liquid, or exploring the effect of temperature or pressure on density.
Conclusion
The experiment successfully determined the density of a solid and a liquid and compared the values with their known densities. The results showed that the calculated densities were in agreement with the known densities, indicating that the experimental procedure was accurate and reliable.
The experiment provided hands-on experience in measuring mass and volume, calculating density, and analyzing results, which are important skills for students in the field of chemistry.
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
Back to the Basic Laboratory Operations experiments page.
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.