Written by J.A Dobado | Last Updated on April 22, 2024
What is a solution?
A solution is a homogeneous mixture of pure substances in which there is no precipitation. It is also called molecular dispersion. A solution consists of a solvent and one or more solutes (the proportions of which vary from one solution to another). In contrast, a pure substance has a constant composition. The solvent is the medium in which the solutes dissolve.
A solution is different from a suspension, colloidal dispersion or an emulsion. The essential difference is in the size of the solute particles. The particle diameter is less than 100 Å. It is characterized by being homogeneous and permanently stable.
Suspension
Also called coarse dispersion, it occurs when the particle diameter is greater than 10,000 Å. It is a heterogeneous mixture and is characterized by its lack of stability (e.g. earth/water).
Colloidal dispersion
The diameter of the particles varies between 100-10,000 Å. These particles are called micelles. It is still a heterogeneous mixture but it is necessary to observe its heterogeneity under the microscope.
The stability is much higher, although we can make it lower by centrifugation.
Emulsion
It occurs when the dissolved substance is also a liquid (oil and water).
Conventions to indicate the solute
- when in a solution there is a solid or gas and a liquid, the latter will be the solvent and either of the first two the solute.
- when the solution is formed by two liquids the solute will be the one that is in smaller proportion.
- when the proportion of the liquids present in a solution is the same, the solvent is the one most commonly used (methanol/water, solvent is water)
Types of solutions
There are three criteria for classifying solutions:
- By the number of components of the solution: binary if they have 2 components, ternary with 3 components, etc.
- By its physical state: a binary solution gives rise to 9 possible solutions, three in gaseous state, three in liquid and three in solid.
| Solute (solvent) | Example |
| gas (gas) | Air:O2 in N2 |
| liquid (gas) | Mist: droplets of H2O in air |
| Solid (gas) | Smoke: particles in air Iodine sublimated in N2. |
The last two are not true solutions because they are not homogeneous.
| Solute (solvent) | Example |
| gas (liquid) | O2 / H2O |
| liquid (liquid) | Gasoline; EtOH / water |
| Solid (liquid) | Glucose / water |
If O2 reacted with water it would no longer be a solution.
| Solute (solvent) | Example |
| gas (solid) | H2 / Pt |
| liquid (solid) | Hg / Cu |
| Solid (solid) | Cu / Ni; Au / Cu |
Solid solutions are alloys and have been obtained in a liquid state and are therefore not true solutions.
- Based on the composition and chemical nature of the components.
If the components of a solution are similar in nature, there will be no specific interactions between them, so it will be an ideal solution.
Example: CCl4 /SiCl4 or benzene / toluene.
If the nature is different, attractive or repulsive interactions may occur between the components.
E.g. Attractive interaction in HNO3 / H2O
Repulsive interaction Et-O-Et / Me-CO-Me
Colligative properties of solutions
Degree of dissociation and dissociation constant of weak electrolytes
The degree of ionic dissociation is the fraction of dissociated molecules or the rate of dissociation (α). The relationship between the van’t Hoff factor “i” and the degree of dissociation “α” of an electrolyte in solution makes it possible to calculate the latter value.
i > 1 van’t Hoff factor increases as the dilution increases:
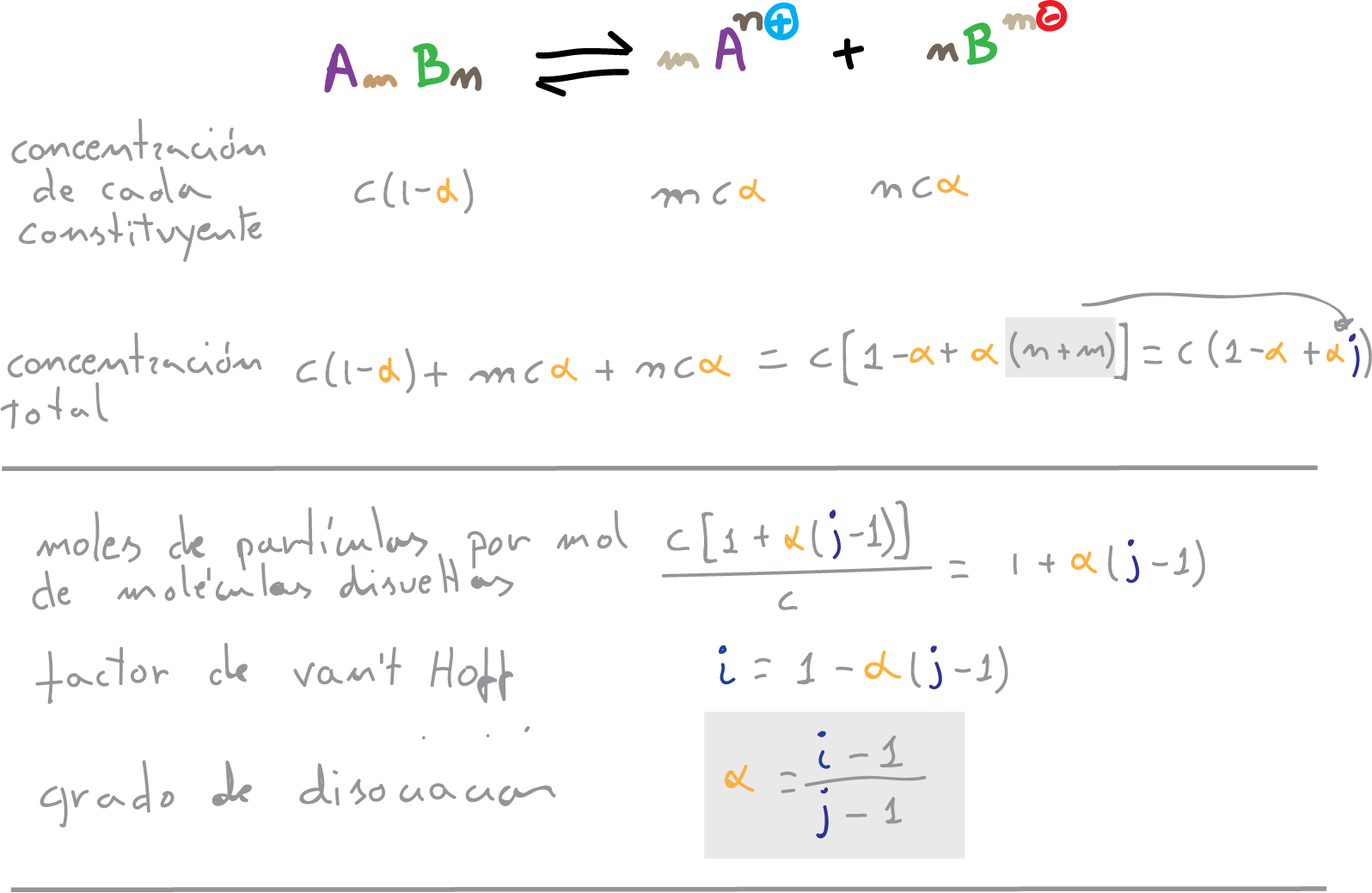
The van’t Hoff factor will be:
i = (c(1-α) + mcα + ncα)/c = 1-α + mα + nα = 1-α + α (m + n)
(infinite dilution i = j)
j = m + n
i = 1 – α + α·j = 1 + α·(j-1)
and the degree of dissociation
α = (i-1)/(j-1)
Values of the standard degree of dissociation are as follows
α > 0.5 strong electrolytes
α < 0.5 weak electrolytes
for example, in the case of potassium chloride:
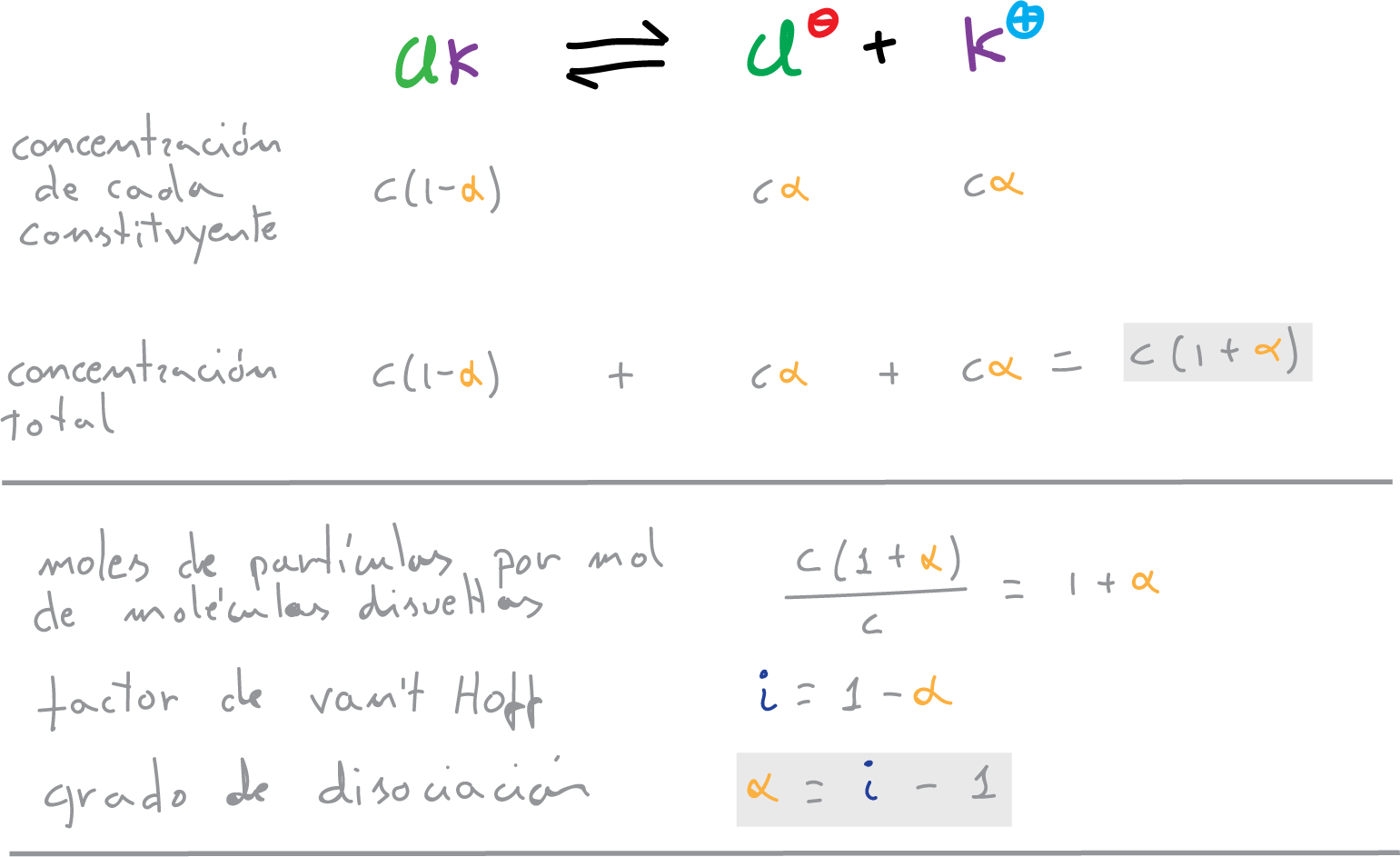
Activity and concentration
If we have a series of ions in a solution, electrostatic interactions, either repulsive or attractive, will occur between them. These interactions will be more intense the higher the concentrations of the ions in the solution. This will cause the actual concentrations of these ions to change, or, in other words, the number of effective particles present in the solution to decrease.
In these concentrated solutions the van’t Hoff coefficient or factor will not be an integer. The need then arises to distinguish between concentration and activity of a solution, which does not have to be proportional to the number of particles, but will depend on the interactions that occur in the solution. Therefore, the activity is equal to the activity coefficient multiplied by the concentration.
a = c·γ ⇒ γ= a/c
(1 > γ > 0)
where a = activity, c = concentration and γ = activity coefficient. Only when the concentration is very dilute, the concentration will be equal to the activity.
Video about Solution
Ions in living systems
There are a number of cations and anions that are found abundantly in living organisms:
Cations: Na+, K+, Mg2+, Ca2+, Fe2+, Fe3+, etc…
Anions: Cl–, HCO3–, H2PO4–, HPO42-, etc…
Potassium and magnesium ions are found mainly in the cellular fluid, while calcium and sodium ions are found in the intercellular fluid.
Small amounts, called trace amounts, of many other metal cations must also be present.
Fe2+ hemoglobin O2/CO2 transport
Fe2+/Fe3+ cytochromes oxidative phosphorylation
Cu2+, Zn2+, Co2+, Mn2+ enzymatic function
Other ions cannot be in living systems as they would cause poisoning, such as Hg2+ and Pb2+, as they react with the sulfur atoms of proteins involved in vital functions of the organism, disturbing them and preventing them from performing their normal functions.
FAQ
What is a solution and how is it formed?
A solution is a homogeneous mixture at the molecular or ionic level of two or more pure substances that do not react with each other, whose components are in varying proportions. It can also be defined as a homogeneous mixture consisting of a solvent and one or more solutes.
What is a solution and examples?
For example: sugar (solute) + water (solvent) = sugar water (solution). The combination of a solute and a solvent is also called a solution. Water is known as the universal solvent since there are many substances that can be diluted in it.