Written by J.A Dobado | Last Updated on April 22, 2024
On an industrial scale
Mainly chlorides, they are generally prepared by direct halogenation of hydrocarbons at high temperatures, by means of free radicals.
Although most of the time mixtures containing isomers and varying amounts of halogen are obtained. However, industrially they are useful, since these mixtures can be used as such.
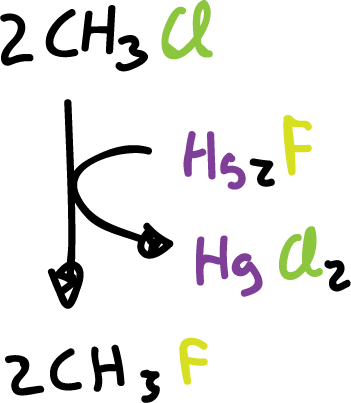
Many fluorides, industrially, are obtained by substitution of the halogen in a halide, using inorganic fluorides as reagents.
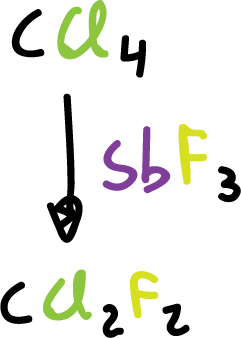
At laboratory scale
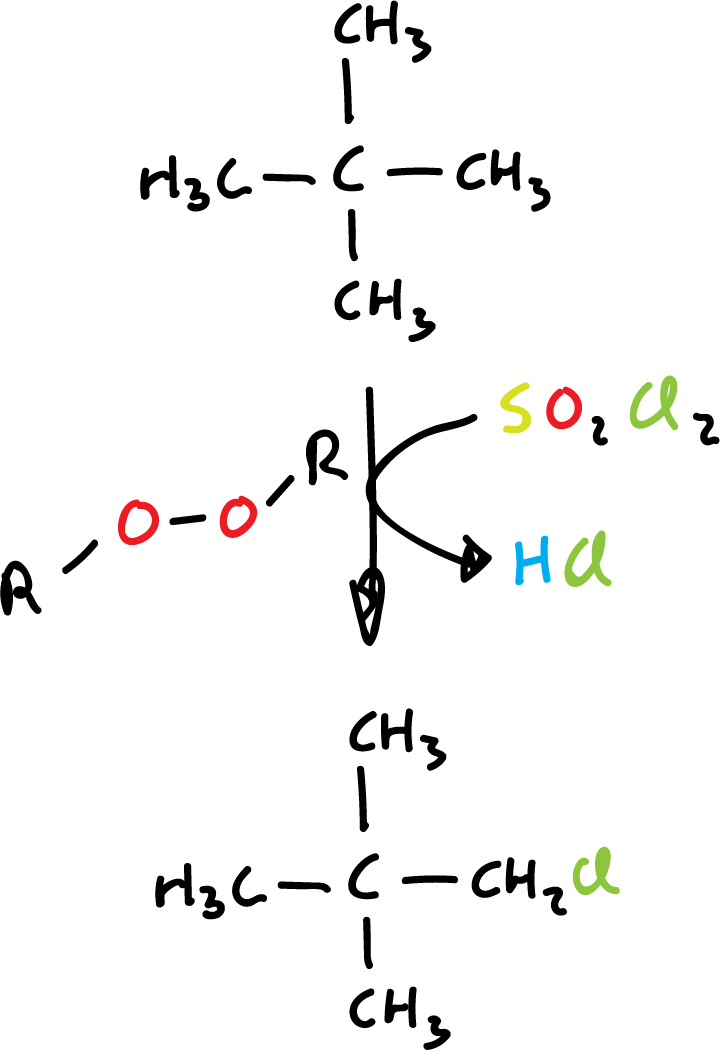
In the laboratory, sulfuryl chloride (SO2Cl2) or thionyl chloride (SOCl2) is used as a chlorinating agent, using an organic peroxide as a radical initiator.
The synthesis of haloalkanes can be performed using different methods listed below:
Synthesis of haloalkanes by the reaction of alcohols with hydrogen halide
The least reactive hydrogen halide (XH) is (HCl), as it requires the presence of zinc chloride (ZnCl2) to react the primary alcohols.
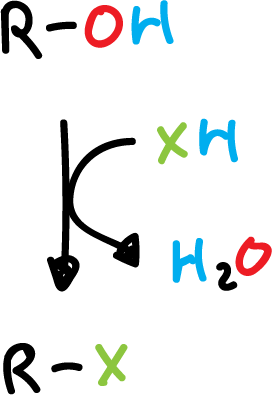
However, terbutanol (t-BuOH) reacts readily by stirring with HCl at room temperature.
In general, the order of reactivity of alcohols is: tertiary (3º) > secondary (2º) > primary (1º).
Examples of synthesis of alcohols with hydrogen halide
1) Example of cyclohexanol with hydrogen bromide to yield bromocyclohexane.
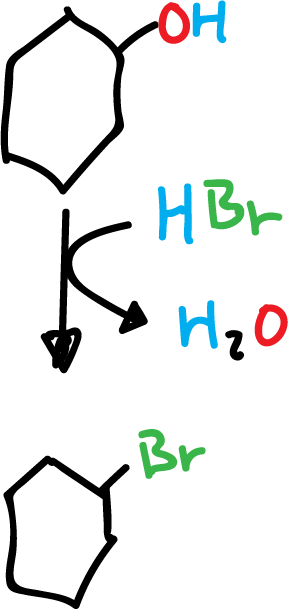
2) Example of propanol with hydrochloric acid and zinc chloride to give 1-chloropropane.
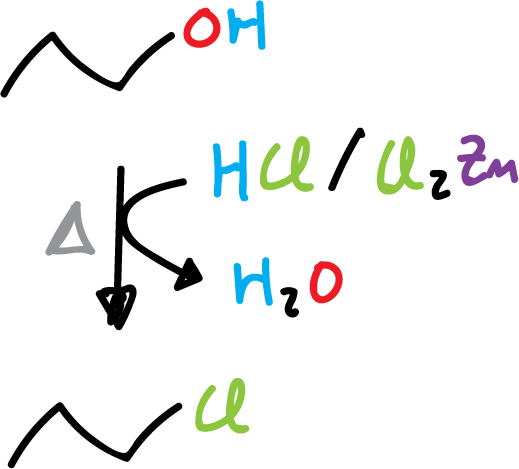
3) Example of terbutanol with concentrated hydrochloric acid to yield tert-butyl chloride.
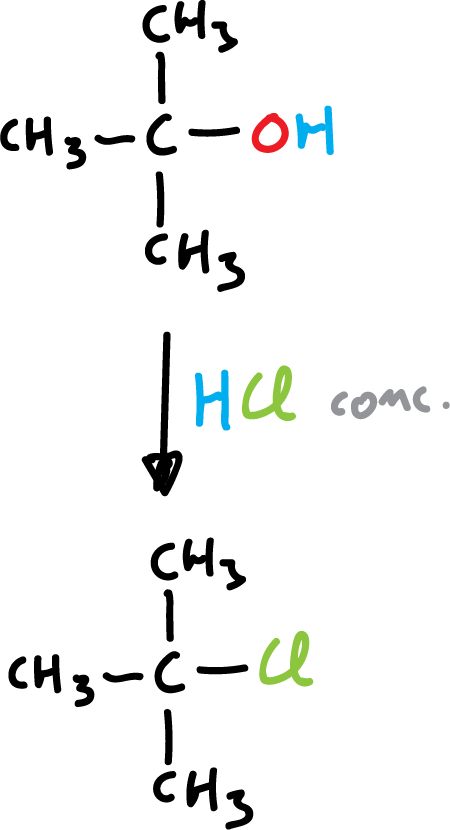
Synthesis of haloalkanes by reaction with thionyl chloride.
The thionyl chloride (SOCl2) is a very useful reagent for converting alcohols to alkyl chloride, since the reaction products are alkyl chloride, HCl and SO2. Thus, these are gaseous and can be easily removed from the reaction crude.
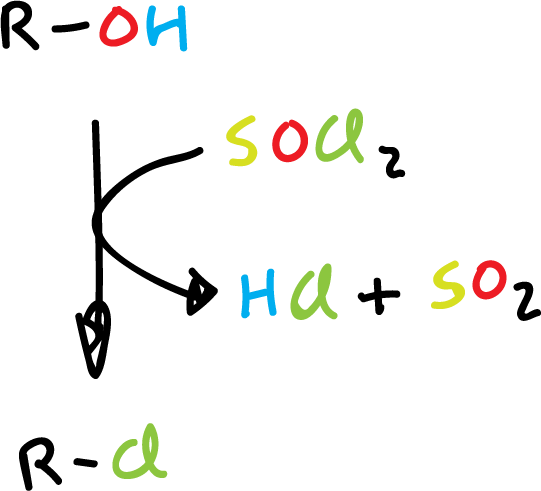
Synthesis of haloalkanes from alkenes and hydrogen halides.
An alkene can be converted to alkyl halide by reaction with hydrogen chloride, hydrogen bromide or hydrogen iodide. The reaction is carried out by passing dry gaseous hydrogen halide through the alkene.
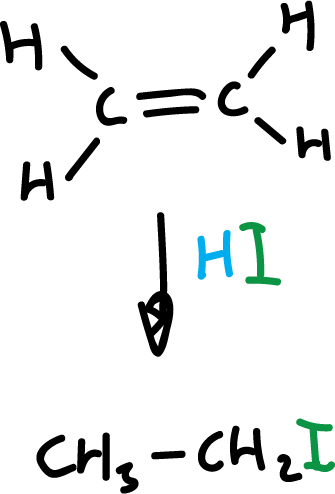
When the alkene is asymmetric, the addition takes place in a particular orientation.
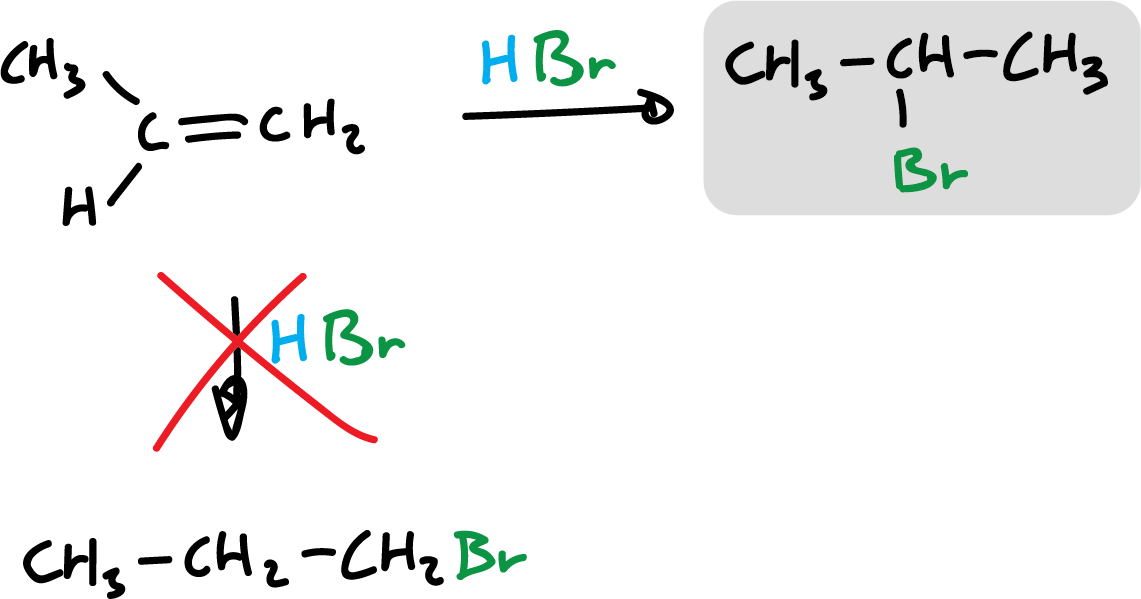
The reaction can go two ways, but only one is favored. This preference as to the position or orientation in which molecules react with each other is called regioselectivity.
The regioselectivity in this particular reaction, addition of hydrogen halides, has long been known and follows the so-called Markovnikov’s rule.
“In the addition of a hydrogen halide to an asymmetric alkene, the halogen is added to the least hydrogenated carbon, that is, to the carbon atom that is most subject to the influence of the other carbon atoms.”
or
“In the ionic addition of an acid to the double bond of the alkene, the hydrogen of that one bonds to the carbon atom having the highest number of hydrogens.”
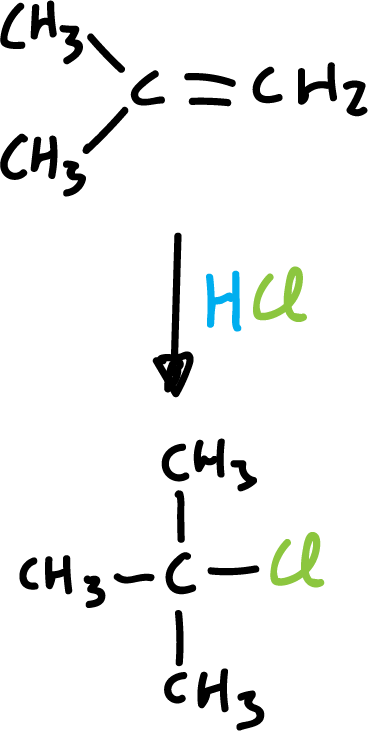
These additions are discussed in more detail when studying alkene reactions. They occur with the formation of carbocations in the reaction.
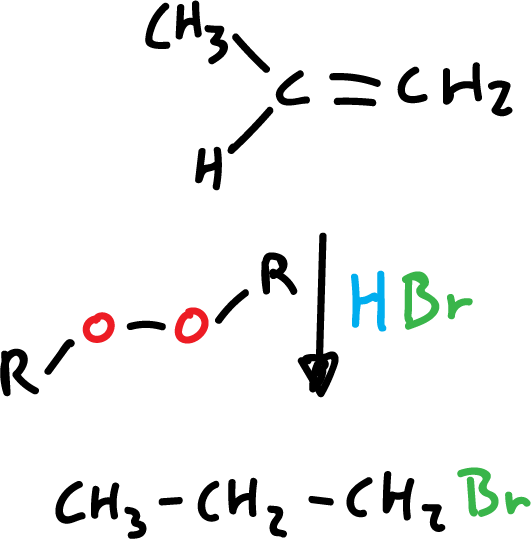
In the case of HBr addition in the presence of organic peroxides, it involves the participation of free radicals and the orientation is different (anti-Markovnikov).
Synthesis of haloalkanes from alkenes and halogens
Chlorine (Cl2) and bromine (Br2) are added to alkenes and transform them into saturated compounds containing two halogens attached to adjacent carbons. However, iodine (I2) does not react.
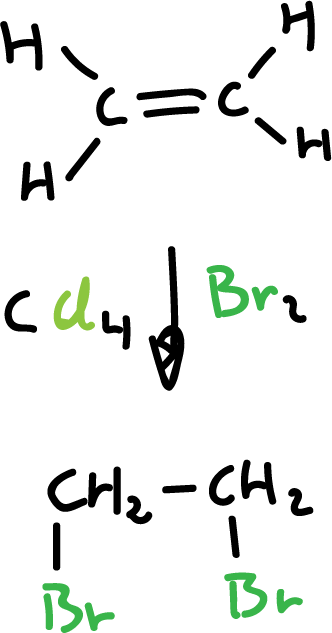
The reaction is carried out with an inert solvent such as carbon tetrachloride (CCl4) and proceeds rapidly at room temperature.
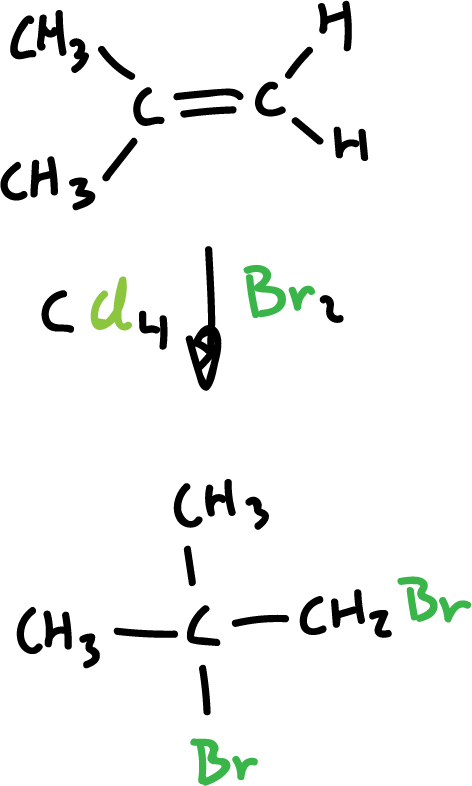
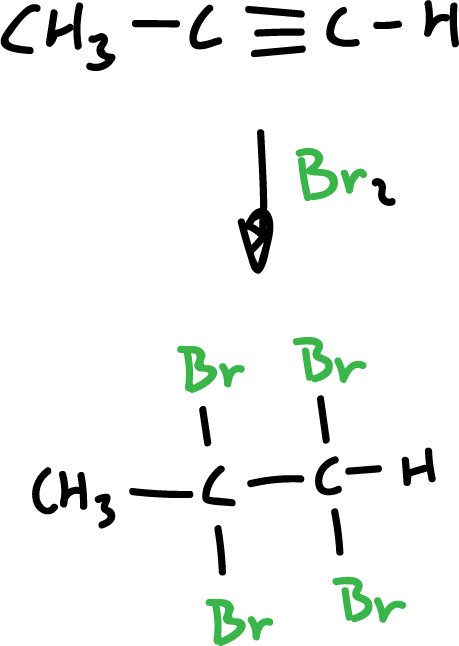
Synthesis of haloalkanes from alkynes and halogens
Halogens, too, can be added to alkynes, except that in this case two moles of halogen are consumed.
Synthesis of haloalkanes from other halogens
Alkyl iodides are usually prepared from another halide by treatment with sodium iodide (NaI) in acetone (CH3COCH3).
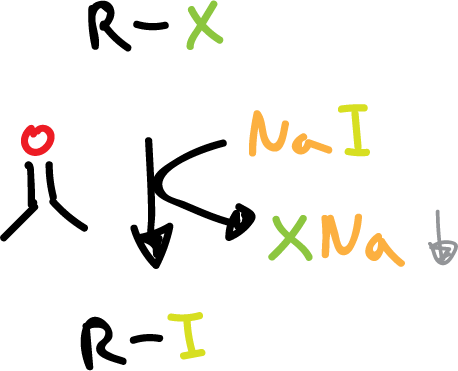
Articles on the website