Written by J.A Dobado | Last Updated on April 22, 2024
What is Thin layer chromatography (TLC)?
Thin layer chromatography (TLC) is one of the most popular variants of liquid-solid chromatography,[1] and its most frequent uses are:
- To determine the components of a mixture.
- To monitor the progress of a reaction.
- Follow the course of a reaction.
- Determine the appropriate conditions for the separation of mixtures in column chromatography (CC).
- To Follow the development of a chromatography column.
Adsorption principle
The sample is applied on the layer and adsorbed on the surface of the material by the action of electrostatic forces (van der Waals forces, hydrogen bonds, inductive effects, etc.). Then, when the plate is exposed to a flow of solvent by capillary action, a competition of bonds between the active sites of the adsorbent and the substance with the solvent is initiated.
Adsorbents
The most commonly used adsorbents in thin layer chromatography (TLC) are the following:
- Silica gel (SiO2): It is used in 80% of the separations.
- Alumina (Al2O3) (acidic, neutral or basic).
- Natural diatomaceous earth or Kieselguhr.
- Cellulose (native or micro-crystalline).
- Polyamide.
In most cases, in an organic chemistry laboratory, silica gel is used as a support (solid phase). These adsorbents are characterized by particle size (pore volume, pore diameter, surface area, homogeneity) and purity.
The adsorbent is deposited on a plate that acts as an inert support and is usually made of glass, aluminum and polyester.
Although the use of commercial chromatofolios is becoming more and more widespread, thin layer chromatography (TLC) plates can also be prepared using glass slides. They can be prepared by introducing two slides together, clean and dry, in a suspension of 35 % silica gel in AcOEt, containing CaSO4 as an adhesive.
Immersion in the adsorbent suspension is carried out with the aid of forceps. The silica gel forms a film on each side of the slides that is exposed to the suspension. Evaporation of the solvent leaves the plate ready for use.
Determining Rf
To help identify the products found in a mixture, the so-called retention factor Rf (ratio of front) is often used. The way to calculate the Rf is illustrated in the figure below:
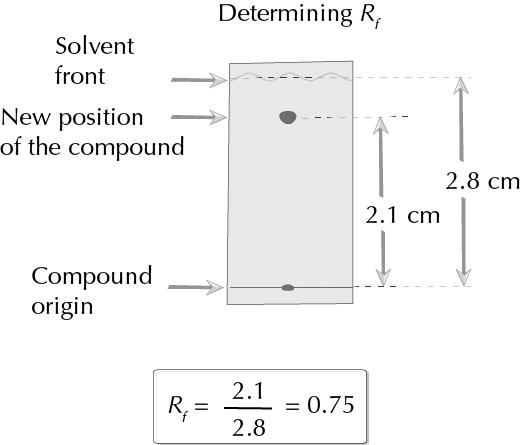
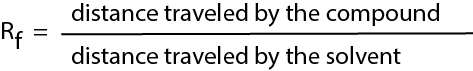
Description of materials and procedures used in this technique
The materials used in this technique are detailed below:
- TLC plate
- Capillary tubes and Pasteur pipettes.
- Eluents.
- Developing tank (chromatography tank).
- Developer.
TLC plate.
Glass, plastic or aluminum foils (chromatoplates) are used as adsorbent support. Conventional thin layer chromatography (TLC) plate sizes are usually 20 x 20, 10 x 20 and 5 x 2 (in cm). There are plates that contain a fluorescence indicator (F254 o F366).[2] The plates can also be used as a fluorescence indicator.
Capillary tubes and Pasteur pipettes.
The sample is applied dissolved on the TLC plate depending on whether the goal is preparative or analytical. For preparative purposes, the sample is applied dissolved and with the aid of a Pasteur pipette, this is called band application. If the technique is intended for analytical purposes, the dissolved sample is applied with the aid of a capillary tube.
In this case it is called spot application. The sample should be applied at a certain distance from the edge of the TLC plate. The height and the precise place where the sample is placed can be marked with a pencil, taking care not to damage the adsorbent.
Eluent selection
It is recommended to choose a solvent in which the components of the mixture have an average Rf around 0.3-0.5. The search for the ideal eluent requires testing with several solvents of different polarity or with mixtures.
When a compound elutes at Rf below 0.2 or above 0.7, it may happen that what appears to be a single compound is actually a mixture of several compounds. In these cases, it is necessary to change to another more or less polar eluent, respectively.
For low polar compounds, which move from the origin very easily, apolar eluents such as hexane should be used. In the case of compounds of medium polarity, it is advisable to use hexane/ethyl acetate or hexane/diethyl ether mixtures in different proportions. More polar products, which are highly retained on the adsorbent, require a more polar solvent such as CH2Cl2/MeOH mixtures in different proportions.
One way to check if the eluent is suitable is to take a sample of the mixture to be chromatographed and dissolve it in the chosen mixture of solvents and to apply it with a capillary tube on a TLC plate and observe the result of the diffusion of the sample.
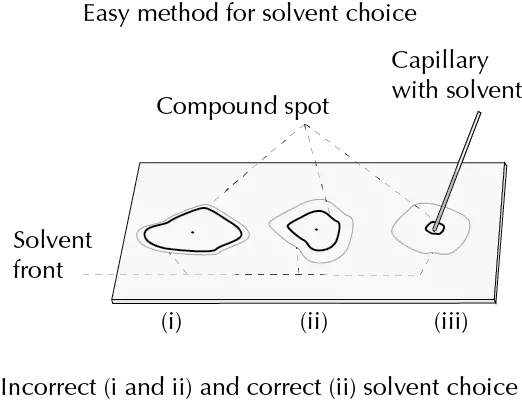
If it is detected that the stains corresponding to the product form a circle approximately in the middle of the one formed by the solvent on the plate, the choice is the correct one, if on the contrary it advances very close to the front of the solvent, or it remains close to the point of origin, where the stain was applied, another solvent should be sought.
Developing tank (chromatography tank)
It is a closed container used for the development of TLC plates. Its atmosphere is saturated with eluent vapors. Several types of tanks are available on the market.
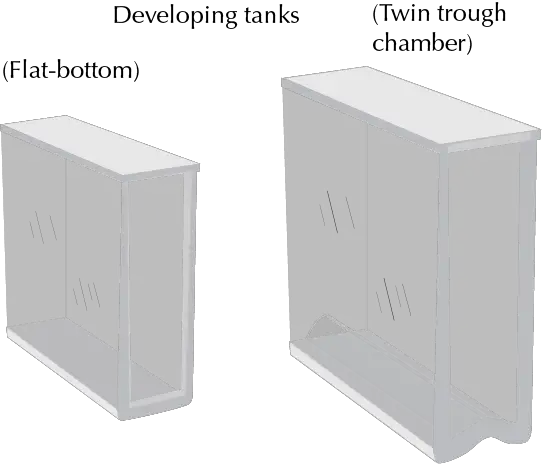
Flat bottom developing tanks with different designs, double compartment tanks (with the advantage of reducing solvent consumption and avoiding residues, as well as allowing the environment to be saturated with solvent vapors other than the eluent), etc.
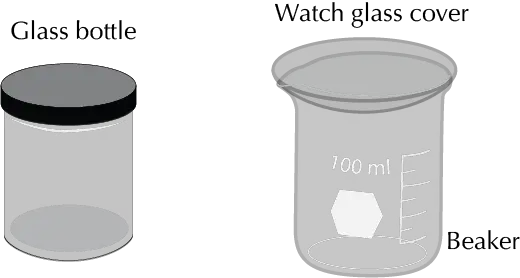
Also, closed flasks and even a beaker covered with a watch glass are used. The tank is filled with the eluent in such a way that the height of the eluent does not reach the area where the products have been placed.
It is usually a good practice to introduce inside the elution tank a piece of filter paper that reaches the top of the tank so that when the eluent rises by capillarity, the tank is saturated with the eluent vapors. This facilitates a correct development of the plate. When the solvent front has reached the upper edge of the plate, it is removed from the tank and the plate is developed.
Detection or visualization (development)
If the sample is not colored, methods that allow us to visualize the components present will be needed. This procedure is known as developing. There are different methods and among the most used we highlight the following:
- Using a 254 nm UV lamp with filter: When using UV indicator plates if the substances have at least one chromophore, darker light green spots and sometimes brighter spots, usually blue, are observed.

| DANGER! «Never look directly at ultraviolet UV light, as it can cause irreparable damage to the eyes.“ |
- Chemical methods: There are many developers for TLC and the most common are listed below:
- H2SO4/EtOH 50 %. It is a universal and cheap developer. Once the TLC plate has been sprayed, it has to be heated either with a hot air dryer or a hot plate so that the stains appear, presenting dark colors.
- KMnO4 (3 g)/K2CO3 (10 g)/water (300 ml). Considered as universal developer, only incompatible with eluents containing amines. The plate acquires the typical color of permanganate and the spots show yellow and/or brownish shades.
- Phosphomolybdic acid (10 g)/EtOH (100 ml). Although expensive, it is applicable to most organic molecules.
- Ninhydrin (0.1 g)/acetic acid (2 ml)/ acetone (100 ml). Mainly used for amino acids and nitrogen compounds.
- Iodine. It is one of the oldest developers still used for a wide variety of organic molecules. By introducing the plate for a few minutes in a tank containing a few iodine crystals, brownish spots are observed which disappear when the plate is removed from the tank, therefore it is recommended to mark the spots with a pencil for later reference.
Developers can be used either by spraying the plate or by immersion in a tank with the developer solution. In the case of spraying the plates, booths are used to concentrate the sprayed developer on the plate and are usually placed in a fume hood so that the aerosol remains are extracted from the workplace.
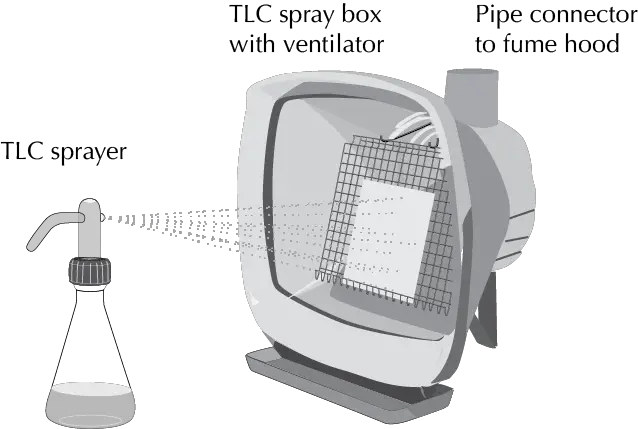

Analytical TLC procedure
- Prepare a solution with a small amount of the sample.
- For 20 x 20 cm TLC plates, cut them with the help of a cutter into plates of a height of approximately one third of the plate and of a variable width depending on the number of samples to be applied.
- Draw a line with a pencil approximately 0.5 cm from the edge of the plate and mark with a dot the places where the sample will be applied with the capillary tube.
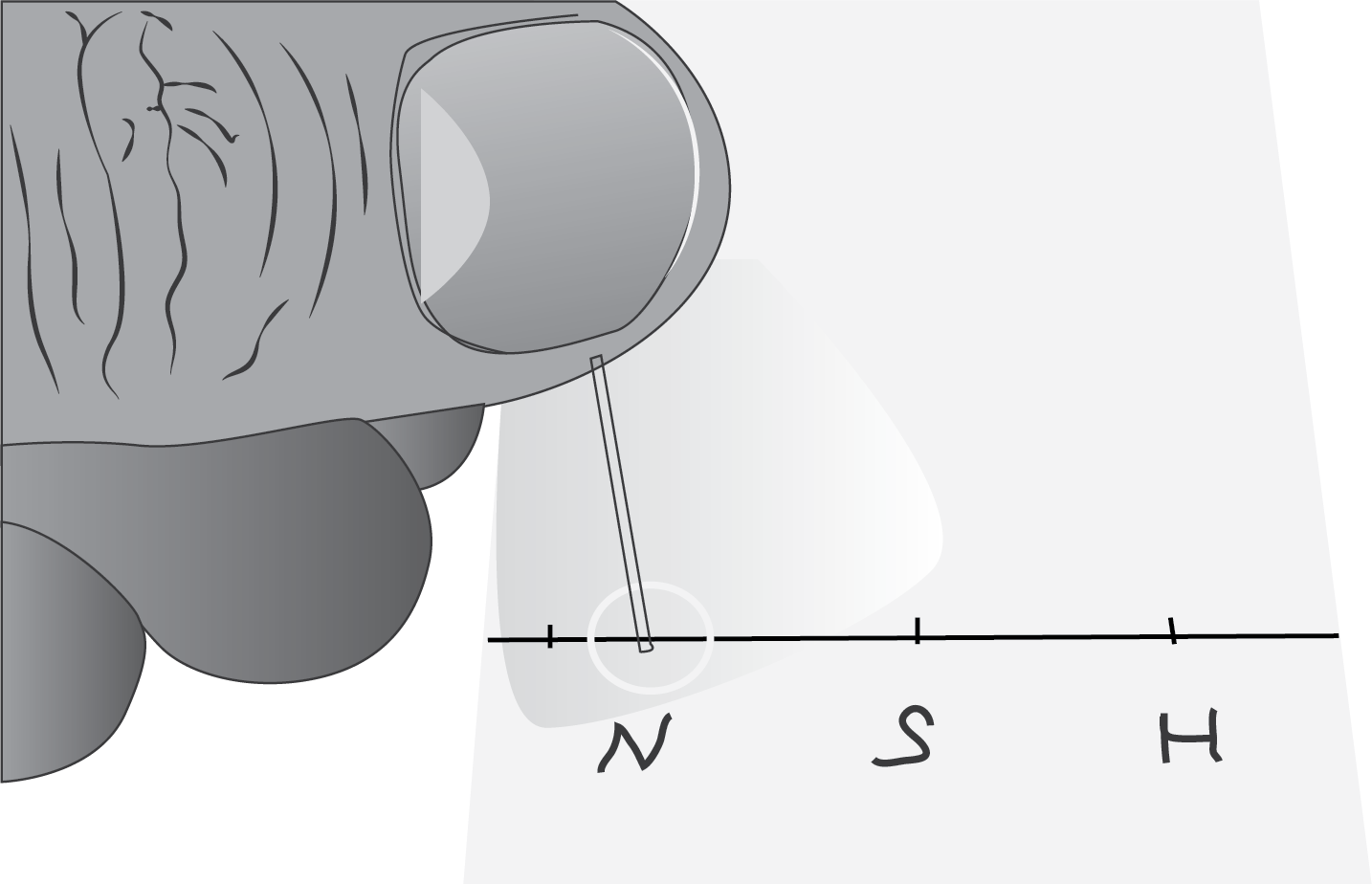
- Wet the capillary tube in the sample solution and transfer the contents to one of the marked spots on the plate. Dry if necessary with an air dryer to prevent the solution from spreading along the surface of the plate before placing it in the developing tank.
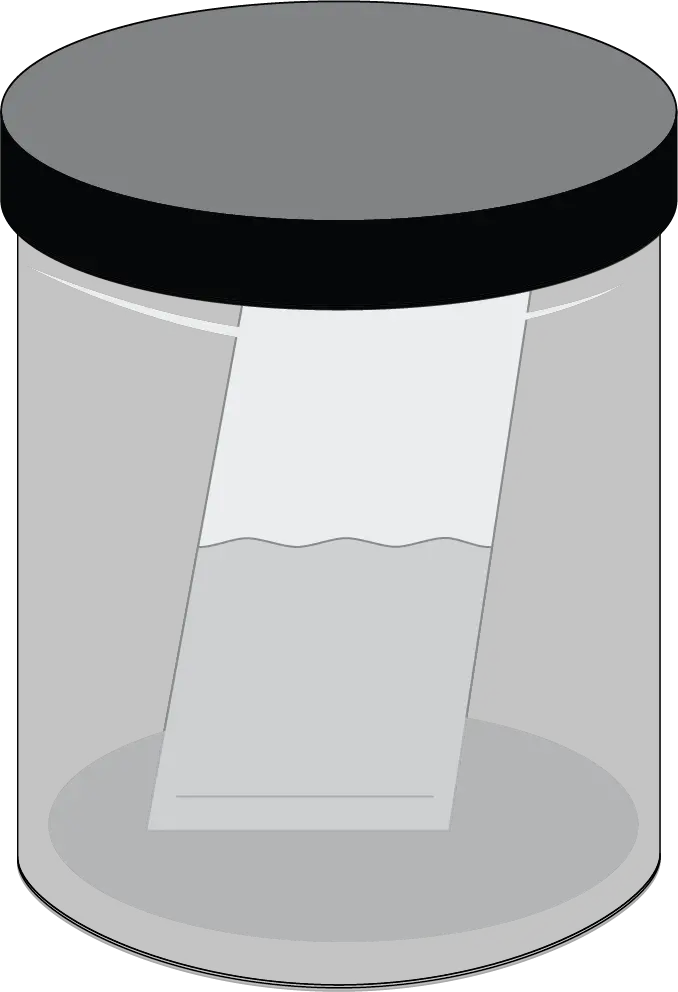
- Insert the plate in the tank and let the eluent rise to the upper edge.
- Remove it from the tank and proceed with the development.
References and notes
- [1] Izmailov and Scraiber used glass slides, in 1938, to place very thin layers of alumina and then applied plant extracts, thus originating the first form of «thin layer chromatography (TLC)». Egon Stanl (1956) gave the name Thin Layer Chromatography (TLC). He standardized procedures, equipment and adsorbents, popularizing this simple, efficient and low-cost technique.
- [2] The number appearing as a subscript indicates the excitation wavelength of the indicator used.
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2