Written by J.A Dobado | Last Updated on April 22, 2024
Objective
The objective of this experiment is to prepare 1-iodobutane by carrying out the Finkelstein reaction on 1-bromobutane, and to carried out a reaction with SN2 mechanism.
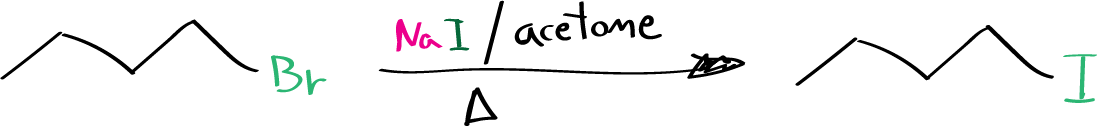
Background
Alkyl iodides can be synthesized through nucleophilic substitution of the corresponding bromides using a solution of sodium iodide in acetone. This reaction follows an SN2 mechanism, and the equilibrium position of the halide exchange is shifted towards iodide formation by the precipitation of the sparingly soluble sodium bromide. The Finkelstein reaction is the name given to this process, which is equally effective for preparing iodides from chlorides. However, primary halides undergo this reaction more efficiently than secondary or tertiary halides due to the SN2 mechanism.
Experimental procedure
In a 250 mL round-bottom flask with magnetic stirring, dissolve 15 g (0.1 mol) of sodium iodide in 80 mL of acetone. Add 1-bromobutane to the flask and reflux the mixture over a water bath for 20 minutes. After heating, remove the flask from the bath and allow it to cool to room temperature.
| DANGER! “Alkyl halides, particularly iodides, are toxic and have been linked to cancer. Avoid skin contact or inhalation.” |
Set up the distillation apparatus and distil approximately 60 mL of acetone from the mixture. Next, cool the residue to room temperature using an ice bath and add 50 mL of water. Extract the product using 25 mL of diethyl ether, and separate the organic phase.
To remove any coloration due to liberated iodine during the reaction, wash the organic phase with 10 mL of saturated aqueous sodium bisulfite. Dry the solution with MgSO4, by gravity filtration, and remove the solvents using a rotary evaporator without any external heating.
The crude 1-iodobutane can be purified by distillation at atmospheric pressure. Collect the fraction boiling at about 125-135 ºC while minimizing decomposition by placing a short length of bright copper wire in the distilling flask. Alternatively, the product may be distilled at reduced pressure, collecting the fraction boiling at about 60-65 ºC.
Record the weight of the obtained product and calculate the yield.
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Vogel, A.I., Furniss, B.S., Hannaford, A.J., Tatchell, A.R., and Smith, P.W.G. (1989). Vogel’s Textbook of Practical Organic Chemistry (Vogel’s Textbook series). Longman. ISBN: 9780470214145