Written by J.A Dobado | Last Updated on April 22, 2024
Objective
The objective of this experiment is to provide familiarity with a reduction reaction using sodium borohydride (NaBH4) as the reducing agent. The experiment will utilize 4-t-butylcyclohexanone as the starting material for the reaction.
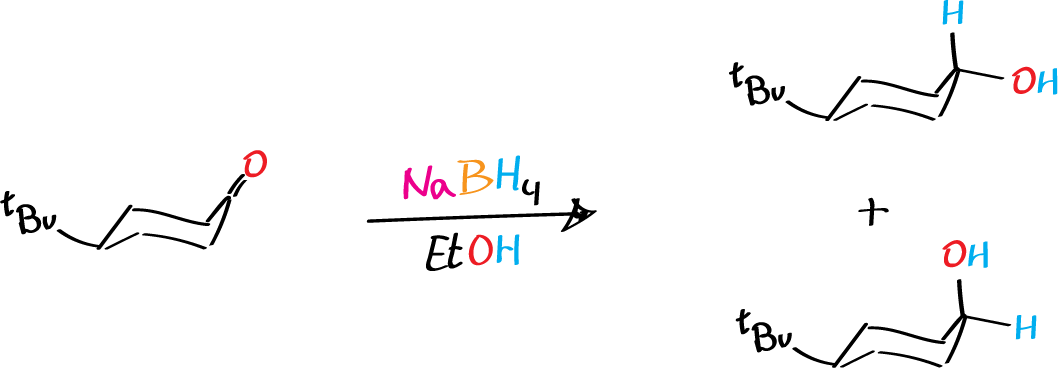
Background
Unsymmetrical ketones can be converted to sp3 secondary alcohols by reducing the sp2 carbonyl group. This reaction creates a new asymmetric center, and the stereochemical outcome depends on the side of the carbonyl group that is attacked by the reagent. However, it is only possible to distinguish between the two possible secondary alcohol products if there is another stereochemical ‘marker‘ present in the starting ketone.
In this experiment, the reduction of 4-t-butylcyclohexanone with an excess of sodium borohydride is carried out. The bulky t-butyl group remains in the equatorial position in the chair conformation of the six-membered ring. Therefore, the two possible alcohol products will have the OH group axial (cis to the t-butyl group) or equatorial (trans to the t-butyl group). The resulting isomeric alcohols are formed in unequal amounts, and their ratio can be determined using gas chromatography (GC).
Experimental procedure
A) Preparation of cis– and trans-4-t-butylcyclohexanol
Dissolve the 4-t-butylcyclohexanone in 20 mL of EtOH in a 50 mL Erlenmeyer flask. Stir the solution magnetically at room temperature and add the sodium borohydride, NaBH4 in small portions over 15 minutes. If necessary, rinse in the last traces of sodium borohydride, NaBH4, with 5 mL of ethanol. Allow the mixture to stir at room temperature for a further 30 minutes, and then pour it into a 250 mL beaker containing 50 mL of ice-water. Slowly add 10 mL of dilute hydrochloric acid and stir the mixture for 10 minutes. Transfer the mixture to a 250 mL separatory funnel and extract the product with 2×50 mL portions of diethyl ether. Dry the combined ether extracts over MgSO4. Filter and retain 1 mL of the ether filtrate in a sample tube for analysis by GC. Evaporate the remainder of the filtrate on the rotary evaporator and recrystallize the solid residue from light petroleum. Record the yield, m.p. and IR spectrum of the product after a single recrystallization. Record an IR spectrum of the starting ketone for comparison.
B) Gas chromatography (GC) analysis
The products obtained (alcohols) are formed in unequal amounts, and their ratio can be determined using GC. To analyze the crude reaction product, an ethereal solution will be subjected to chromatography using a Carbowax® column. The goal is to determine the ratio of cis to trans isomeric alcohols based on the relative peak areas. The cis-isomer is expected to have a shorter retention time.
To verify that the reduction reaction has proceeded to completion, a reference sample of 4-t-butylcyclohexanone will also be run alongside the product sample.
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Vogel, A.I., Furniss, B.S., Hannaford, A.J., Tatchell, A.R., and Smith, P.W.G. (1989). Vogel’s Textbook of Practical Organic Chemistry (Vogel’s Textbook series). Longman. ISBN: 9780470214145