Written by J.A Dobado | Last Updated on April 22, 2024
Objective
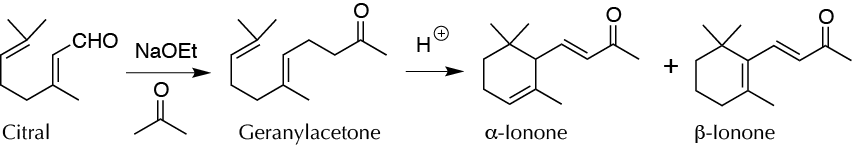
To produce α- and β-ionone (violet perfume) from citral. These are cyclic terpenoid that occur in many essential oils.
Background
Citral (3,7-dimethyl-2,6-octadienal) is the main constituent of the essential oil of lemon grass (citronella). This natural product readily undergoes aldol condensation with acetone to give geranylacetone, which can be converted by cyclization under acidic conditions into a mixture of α- and β-ionones used as an artificial violet flavor. The nature of the catalyst, its concentration, and its temperature play decisive roles in the ratio between the two ionones.
Experimental procedure
A) preparation of geranylacetone
In a 100 ml round-bottom flask, prepare a solution of freshly distilled citral (5 g, b.p. = 91–93 ºC at 2.6 mm Hg) in dry acetone (20 ml). Cool the mix ture to −5 ºC (ice/salt), and add dropwise a solution of sodium ethoxide prepared from 230 mg of sodium and 5 ml of absolute EtOH with magnetic stirring. Continue stirring for another 10 min at −5 ºC, and afterward, neutralize the excess alkali with stirring by adding a solution of tartaric acid in water, prepared from 900 mg tartaric acid in 25 ml deionized water. Remove excess acetone and EtOH in a rotary evaporator. Isolate geranylacetone from the remaining aqueous solution and extract with diethyl ether (2 × 5 ml).
Combine the organic extracts and dry over Na2SO4 anhydrous, remove the desiccant by gravity filtration, and the evaporation generates a liquid that is composed mainly of ψ-ionone. Weigh and calculate the yield. Purify by vacuum distillation (b.p. = 145–150 ºC at 12 mm Hg).
B) Synthesis of α- and β-ionone
To a 100 ml round-bottom flask containing 15 g of a solution of phosphoric acid 85%, add dropwise 2 g of ψ-ionone with magnetic stirring. After the addition, maintain the stirring at r.t. for 1 h. After this time, pour the reaction crude into a 100 ml beaker containing 50 ml of cold water, and then extract the reaction mixture with diethyl ether (2 × 20 ml). Wash the combined ether extracts several times with water (until no acid pH reaction ocurrs). Dry the organic layer over Na2SO4 anhydrous, separate the desiccant by gravity filtration, and remove the solvent on a rotary evaporator. Weigh the resulting liquid and calculate the yield.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Citral | 152.23 | - | 229 | 0.888 |
| α-Ionone | 192.30 | - | 259-263 | 0.930 |
| β-Ionone | 192.30 | -35 | 126-128 | 0.945 |
| Geranylacetone | 192.3 | <25 | 114-116 | 0.900 |
| H3PO4 | 98.00 | 40 | 158 | 1.685 |
| Acetone | 58.08 | -94 | 56 | 0.791 |
| L-(+)-Tartaric acid | 150.09 | 170-172 | - | - |
| Diethyl ether | 74.12 | -116 | 34.6 | 0.71 |
| Na2SO4 | 142.04 | 884 | - | 2.630 |
| Na | 22.99 | 97.8 | 883 | 0.968 |
| EtOH | 46.07 | -114.1 | 78.5 | 0.790 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Citral |  |
| α-Ionone |  |
| β-Ionone |  |
| Geranylacetone | Non-hazardous |
| H3PO4 |  |
| Acetone |   |
| L-(+)-Tartaric acid |  |
| Diethyl ether |   |
| Na2SO4 | Non-hazardous |
| Na |   |
| EtOH |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Citral | WTEVQBCEXWBHNA-JXMROGBWSA-N |
| α-Ionone | UZFLPKAIBPNNCA-BQYQJAHWSA-N |
| β-Ionone | PSQYTAPXSHCGMF-BQYQJAHWSA-N |
| Geranylacetone | HNZUNIKWNYHEJJ-FMIVXFBMSA-N |
| H3PO4 | NBIIXXVUZAFLBC-UHFFFAOYSA-N |
| Acetone | CSCPPACGZOOCGX-UHFFFAOYSA-N |
| L-(+)-Tartaric acid | FEWJPZIEWOKRBE-JCYAYHJZSA-N |
| Diethyl ether | RTZKZFJDLAIYFH-UHFFFAOYSA-N |
| Na2SO4 | PMZURENOXWZQFD-UHFFFAOYSA-L |
| Na | KEAYESYHFKHZAL-UHFFFAOYSA-N |
| EtOH | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- G. A. Poulton, Isomer analysis by spectral methods, Journal of Chemical Education 52 (1975), no. 6, 397, DOI: 10.1021/ed052p397