Written by J.A Dobado | Last Updated on May 2, 2024
What is Meth-Cohn quinoline synthesis?
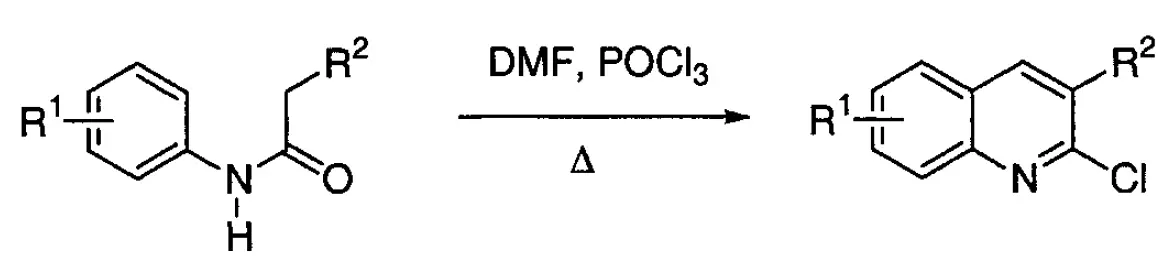
The Meth-Cohn quinoline synthesis entails treating acylanilides with Vilsmeier’s reagent ([(CH3)2NCHCl]Cl) in phosphorus oxychloride (POCl3) solvent under warm conditions, leading to the formation of 2-chloro-3-substituted quinolines.
The Vilsmeier-Haack reaction is a widely used method for the formylation of diverse electron-rich substrates, including aliphatic, aromatic, and heteroaromatic compounds. Although it is primarily used for aromatic formylation, this reaction also provides access to a broad range of heterocyclic systems.
In 1978, Meth-Cohn’s team developed a straightforward procedure in which acetanilide was efficiently converted into 2-chloro-3-quinolinecarboxaldehyde with a yield of 68 %. This quinoline synthesis was later termed the “Vilsmeier approach” by Meth-Cohn or Meth-Cohn quinoline synthesis.
References
- Meth-Cohn, O., and Narine, B. (1978). A versatile new synthesis of quinolines, thienopyridines and related fused pyridines. Tetrahedron Letters, 19(23), 2045-2048. https://doi.org/10.1016/S0040-4039(01)94745-8
- Meth-Cohn, O., Narine, B., and Tarnowski, B. (1979). A versatile new synthesis of quinolines and related fused pyridines. Part II. Tetrahedron Letters, 20(33), 3111-3114. https://doi.org/10.1016/S0040-4039(01)95334-1.
- Meth-Cohn, O., Rhouati, S., and Tarnowski, B. (1979). A versatile new synthesis of quinolines and related fused pyridines. Part III. Tetrahedron Letters, 20(50), 4885-4886. https://doi.org/10.1016/S0040-4039(01)86740-X.
- Meth-Cohn, O., & Tarnowski, B. (1980). A versatile new synthesis of quinolines and related fused pyridines. Part IV.1 A simple one-pot route to pyrido[2,3-b]quinolin-2-ones from anilides. Tetrahedron Letters, 21(38), 3721-3722. https://doi.org/10.1016/S0040-4039(00)78756-9
- Meth-Cohn, O., Narine, B., and Tarnowski, B. (1981). A versatile new synthesis of quinolines and related fused pyridines, Part 5. The synthesis of 2-chloroquinoline-3-carbaldehydes. J. Chem. Soc., Perkin Trans. I, 1520-1530. doi: 10.1039/P19810001520
- Meth-Cohn, O., Narine, B., and Tarnowski, B. (1981). A versatile new synthesis of quinolines and related fused pyridines. Part 7. The conversion of acetamidothiophens into thienopyridines. Journal of the Chemical Society, Perkin Transactions I, 1531-1536. https://doi.org/10.1039/P19810001531
- Lindley, J. M., McRobbie, I. M., Meth-Cohn, O., and Suschitzky, H. (1980). Competitive cyclisations of singlet and triplet nitrenes. Part 8. The 1-(2-nitrenophenyl)pyrazoles and related systems. Journal of the Chemical Society, Perkin Transactions I, 982-994. doi: 10.1039/P19800000982
- Hawkins, D., Lindley, J. M., McRobbie, I. M., and Meth-Cohn, O. (1980). Competitive cyclisations of singlet and triplet nitrenes. Part 9. 2-(2-nitrenophenyl)-benzothiazoles and -benzimidazoles. Journal of the Chemical Society, Perkin Transactions I, (11), 2387-2391. doi: 10.1039/P19800002387
- Meth-Cohn, O., and Tarnowski, B. (1982). Cyclizations under Vilsmeier Conditions. In A. R. Katritzky (Ed.), Advances in Heterocyclic Chemistry (Vol. 31, pp. 207-236). Academic Press. https://doi.org/10.1016/S0065-2725(08)60399-2
- Meth-Cohn, O., and Westwood, K. T. (1983). A versatile new synthesis of quinolines and related fused pyridines. Part 11. Conversion of acylanilides into α-iminopyridines. Journal of the Chemical Society, Perkin Transactions I, (11), 2089-2092. doi: 10.1039/P19830002089
- Meth-Cohn, O., and Westwood, K. T. (1984). A versatile new synthesis of quinolines and related fused pyridines. Part 12. A general synthesis of 2-chloropyridines and 2-pyridones. Journal of the Chemical Society, Perkin Transactions 1, (0), 1173-1182. doi: 10.1039/P19840001173