Written by J.A Dobado | Last Updated on May 2, 2024
What is Skraup quinoline synthesis?
The Skraup quinoline synthesis, also known as Skraup reaction or Skraup synthesis, is a chemical process for synthesizing quinoline derivatives from primary aromatic amines, glycerol, and an oxidizing agent in concentrated sulfuric acid H2SO4.
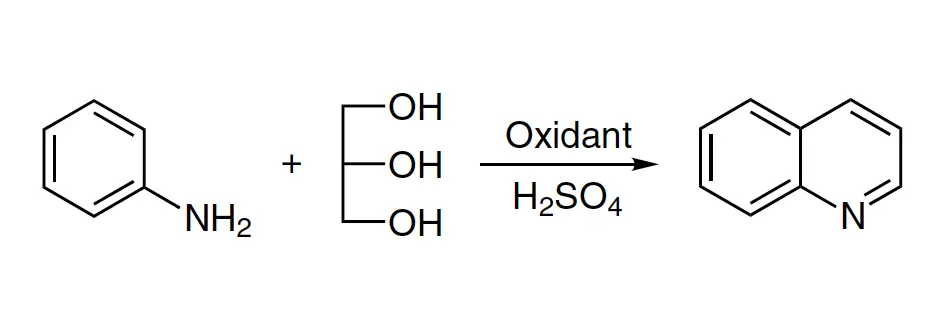
While most primary aromatic amines react under Skraup reaction conditions, the initial Skraup protocol yields a low yield of quinolines. Hence, extensive modifications have been made to the protocol. Among the modified methods, the Doebner-von Miller quinoline synthesis, which uses α,β-unsaturated carbonyl compounds, is the most important.
Various chemicals, including acetic acid, boric acid, FeSO4, thorium, vanadium, or iron oxides, have been added to moderate the reaction as the initial Skraup reaction condition is exothermic with uncontrollable violence.
The Skraup quinoline synthesis can be carried out in protic acid or in the presence of a Lewis acid, such as InCl3, I2, Sc(OTf)3, SnCl4, Yb(OTf)3, and ZnCl2. The ortho– or para-substituted primary aromatic amines form the expected quinolines, whereas the meta-substituted aromatic amines lead to the formation of a mixture.
Aromatic diamines, such as para– or meta-phenylenediamine, fail to give quinoline derivatives in the presence of arsenic acid. However, phenanthrolines can be prepared from the Skraup reaction. It has been suggested that, in the case of using an unstable aromatic amine, a higher yield of quinoline can be obtained from an azo compound of the corresponding aromatic amine.
References
Skraup, Z. H., Ber., 1880, 13, 2086