Written by J.A Dobado | Last Updated on May 2, 2024
What is Lobry de Bruyn-Alberda van Ekenstein transformation?
The Lobry de Bruyn-Alberda van Ekenstein reaction, discovered in 1895, involves the reciprocal conversion of carbohydrates to their isomers in an alkaline solution, with the help of an enediolic intermediate. The reaction is named after its discoverers, and is also known by other names such as the Lobry de Bruyn-Alberda van Ekenstein transformation, Lobry de Bruyn-Alberda van Ekenstein rearrangement, Lobry de Bruyn-Albreda van Ekenstein C-2 epimerization, and Lobry de Bruyn-van Ekenstein transformation. This glycochemical reaction is catalyzed by hydroxide, and is independent of counterions, relying only on the concentration of the base and temperature.
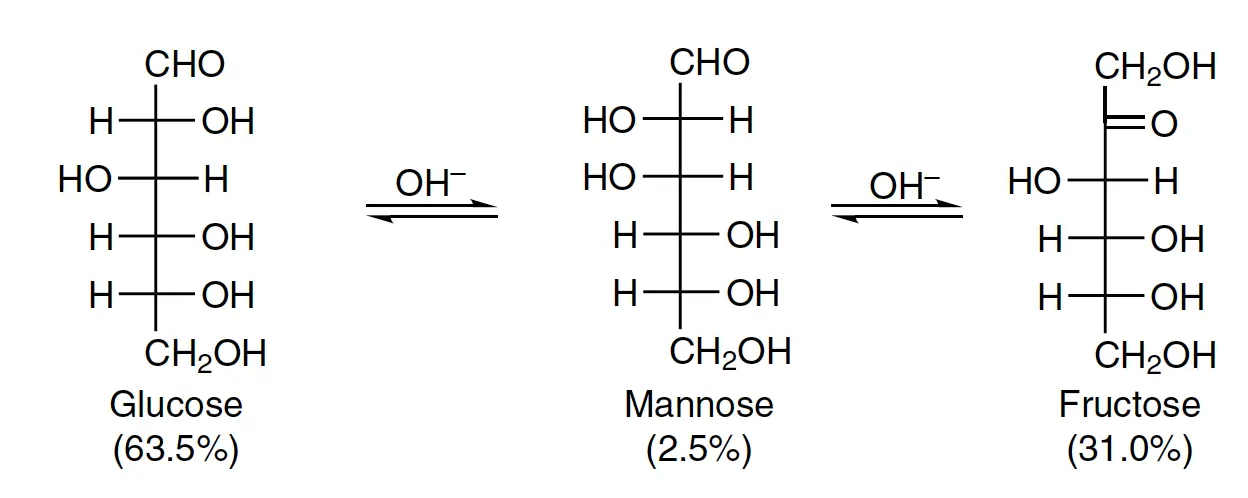
The reaction occurs between glucose, mannose, and fructose, irrespective of the base used, such as KOH, NaOH, NH4OH, Mg(OH)2, Na2CO3, or K2CO3. Similarly, galactose forms an equilibrium with tagatose and talose. Interestingly, sorbose does not undergo this transformation, and glucose treated with lead hydroxide yields only mannose, while fructose does not produce either of its corresponding aldose isomers.
It has been found that this base-catalyzed interconversion of carbohydrates is essentially similar to the isomerase-catalyzed group transfer reaction of glucose-6-phosphate. Additionally, carbohydrates may undergo retro-aldol condensation and degrade into lower analogs under basic conditions.
References
- de Bruyn, C.A.L. (1895), Action des alcalis dilués sur les hydrates de carbone I. (Expériences provisoires). [Action of diluted alkalis on carbohydrates I. (Provisional experiments)] Recl. Trav. Chim. Pays-Bas, 14: 156-165. https://doi.org/10.1002/recl.18950140602
- de Bruyn, C.A.L. and van Ekenstein, W.A. (1895), Action des alcalis sur les sucres, II. Transformation réciproque des uns dans les autres des sucres glucose, fructose et mannose. [Action of alkalis on sugars, II. Reciprocal transformation of the sugars glucose, fructose and mannose into each other] Recl. Trav. Chim. Pays-Bas, 14: 203-216. https://doi.org/10.1002/recl.18950140703
- de Bruyn, C.A.L. and van Ekenstein, W.A. (1896), Action des alcalis sur les sucres, III. Transformation des sucres sous l’influence de l’hydroxyde de plomb. [Action of alkalis on sugars, III. Transformation of sugars under the influence of lead hydroxide] Recl. Trav. Chim. Pays-Bas, 15: 92-96. https://doi.org/10.1002/recl.18960150306
- de Bruyn, C.A.L. and van Ekenstein, W.A. (1897), Action des alcalis sur les sucres. IV: Remarques générales. [Action of alkalis on sugars. IV: General remarks.] Recl. Trav. Chim. Pays-Bas Belg., 16: 257-261. https://doi.org/10.1002/recl.18970160805
- de Bruyn, C.A.L. and van Ekenstein, W.A. (1897), Action des alcalis sur les sucres. V: Transformation de la galactose. Les tagatoses, et la galtose. [Action of alkalis on sugars. V: Transformation of galactose. The tagatoses, and galtose] Recl. Trav. Chim. Pays-Bas Belg., 16: 262-273. https://doi.org/10.1002/recl.18970160902
- de Bruyn, C.A.L. and van Ekenstein, W.A. (1897), Action de l’eau bouillante sur la fructose. [Action of boiling water on fructose] Recl. Trav. Chim. Pays-Bas Belg., 16: 282-283. https://doi.org/10.1002/recl.18970160904
- de Bruyn, C.A.L. and van Ekenstein, W.A. (1899), Action des alcalis sur les sucres. VI. L.e maltose, le lactose et le mélibiose. [Action of alkalis on sugars. VI. Maltose, lactose and melibiose] Recl. Trav. Chim. Pays-Bas Belg., 18: 147-149. https://doi.org/10.1002/recl.18990180502
- de Bruyn, C.A.L. and van Ekenstein, W.A. (1900), Le d-sorbose et le l-sorbose (ψ-tagatose) et leur configuration. [The d-sorbose and l-sorbose (ψ-tagatose) and their configuration] Recl. Trav. Chim. Pays-Bas Belg., 19: 1-11. https://doi.org/10.1002/recl.19000190102