Written by J.A Dobado | Last Updated on April 22, 2024
Objective
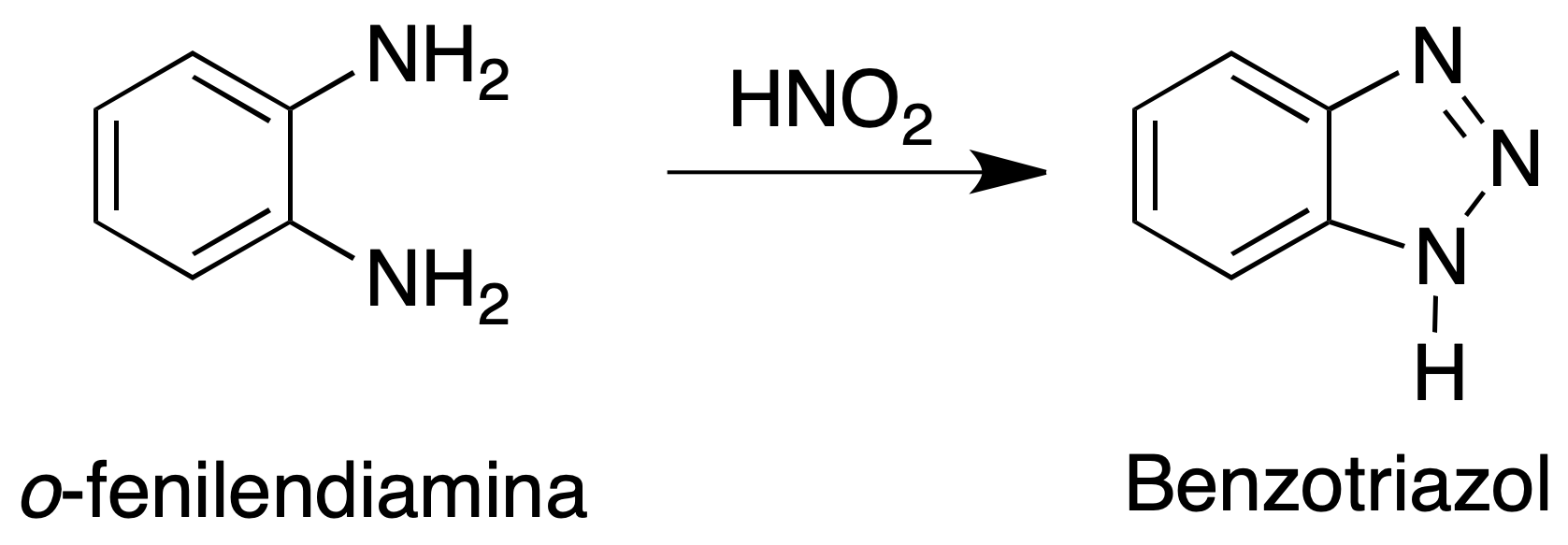
The purpose of this practice is to obtain a simple nitrogenous heterocycle, in this case a triazole (five-membered ring with three nitrogens) fused to a six-membered aromatic ring, benzotriazole.
Background
Benzotriazole is a heterocyclic compound that is used as a starting product in the manufacture of some pharmaceuticals, in photography as a component of developers, or as a component of metal coatings to prevent corrosion, especially of copper and copper alloys. Benzotriazole can be found as an additive in dish detergents, automotive antifreeze, or as an additive in lubricants.
Experimental procedure
Dissolve 5.4 g of o-phenylenediamine in a mixture of 6 g (5.5 ml) of glacial acetic acid and 15 ml of water in a 250 ml Erlenmeyer flask. Heat slightly if necessary to achieve total dissolution of the mixture.
Cool the solution in a cold water bath and add at once a solution of 3.8 g of sodium nitrite in 8 ml of water. Proceed with manual stirring and avoid magnetic stirring. It is observed that the reaction mixture heats up reaching approximately 85 ºC.
At this point it cools down again, and a change in the coloration of the reaction mixture (from dark red to light brown) can be observed.
The cooling-heating-cooling sequence is critical for good performance. Therefore, if the initial or final cooling process is not very efficient, performance decreases.
Stirring is continued for 15 min. and the mixture is placed in an ice bath for another 30 min.
The solid that appears is filtered under vacuum and washed three times with cold deionized water in the Büchner. The solid obtained is benzotriazole. It is recrystallized in water.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| 1H-Benzotriazole | 119.12 | 94-99 | - | - |
| o-Phenylenediamine | 108.14 | 100-102 | 256-258 | 1.030 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| 1H-Benzotriazole |  |
| o-Phenylenediamine |    |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| 1H-Benzotriazole | QRUDEWIWKLJBPS-UHFFFAOYSA-N |
| o-Phenylenediamine | GEYOCULIXLDCMW-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Vogel, A.I., Furniss, B.S., Hannaford, A.J., Tatchell, A.R., and Smith, P.W.G. (1989). Vogel’s Textbook of Practical Organic Chemistry (Vogel’s Textbook series). Longman. ISBN: 9780470214145