Written by J.A Dobado | Last Updated on April 22, 2024
Objective
The purpose of this experiment is the performance of an electrophilic aromatic substitution reaction (SEAr), such as the nitration of methyl benzoate.
Background
An example of an electrophilic aromatic substitution reaction (SEAr) is the nitration of an aromatic ring, using the nitronium ion, ⊕NO2, as an electrophile. The methoxycarbonyl group of methyl benzoate deactivates the ring and directs the substitution at the target position.
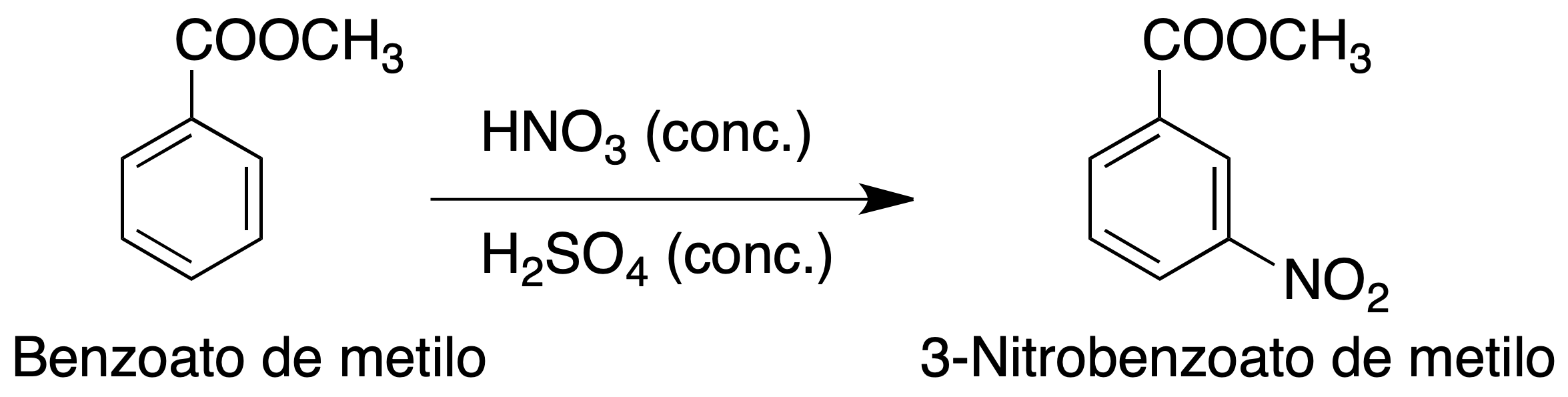
Experimental procedure
Place 1.8 ml (2 g) methyl benzoate in a 25 ml flask and add 4 ml H2SO4 (conc.) while stirring simultaneously. Cool the mixture in an ice bath.
| DANGER! “Nitrous vapors are produced. Perform the entire nitration process in a fume hood.“ |
In another flask put the HNO3 and add the rest of H2SO4, stirring and cooling in ice. Using a Pasteur pipette, to add drop by drop the solution of HNO3 to the solution of methyl benzoate with agitation, maintaining the temperature in a range of 0-10 ºC, with the aid of an ice bath.
The addition requires about 30 min., then the solution is left for 10 min. more at room temperature.
A precipitate is formed by pouring the solution on ice. Filter the methyl 3-nitrobenzoate under vacuum, washing it well in the Büchner with water. Recrystallize from EtOH to obtain an almost colorless solid (estimated yield 55 %)
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| EtOH | 46.07 | -114.1 | 78.5 | 0.790 |
| H2SO4 | 98.08 | 3 | - | 1.80-1.84 |
| HNO3 | 63.01 | - | 120.5 | 1.413 |
| Methyl 3-nitrobenzoate | 181.15 | 78-80 | 279 | - |
| Methyl benzoate | 136.15 | -12 | 198-199 | 1.094 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| EtOH |  |
| H2SO4 |  |
| HNO3 |   |
| Methyl 3-nitrobenzoate | Non-hazardous |
| Methyl benzoate |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| EtOH | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
| H2SO4 | QAOWNCQODCNURD-UHFFFAOYSA-N |
| HNO3 | GRYLNZFGIOXLOG-UHFFFAOYSA-N |
| Methyl 3-nitrobenzoate | AXLYJLKKPUICKV-UHFFFAOYSA-N |
| Methyl benzoate | QPJVMBTYPHYUOC-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Vogel, A.I., Furniss, B.S., Hannaford, A.J., Tatchell, A.R., and Smith, P.W.G. (1989). Vogel’s Textbook of Practical Organic Chemistry (Vogel’s Textbook series). Longman. ISBN: 9780470214145