Written by J.A Dobado | Last Updated on April 22, 2024
Objective
Determine the percentage of ethanol, EtOH, in wine “alcohol by volume” (ABV) using simple distillation and subsequent measurement of the density of the distillate with an alcoholmeter.
Background
One of the characteristics that must be specified in the documentation of alcoholic beverages, e.g. wines, is the percentage (%) of alcohol by volume of EtOH, called alcohol by volume (ABV). In a solution of alcohol and water, it can be measured directly by determining the density with a density meter (alcoholmeter).
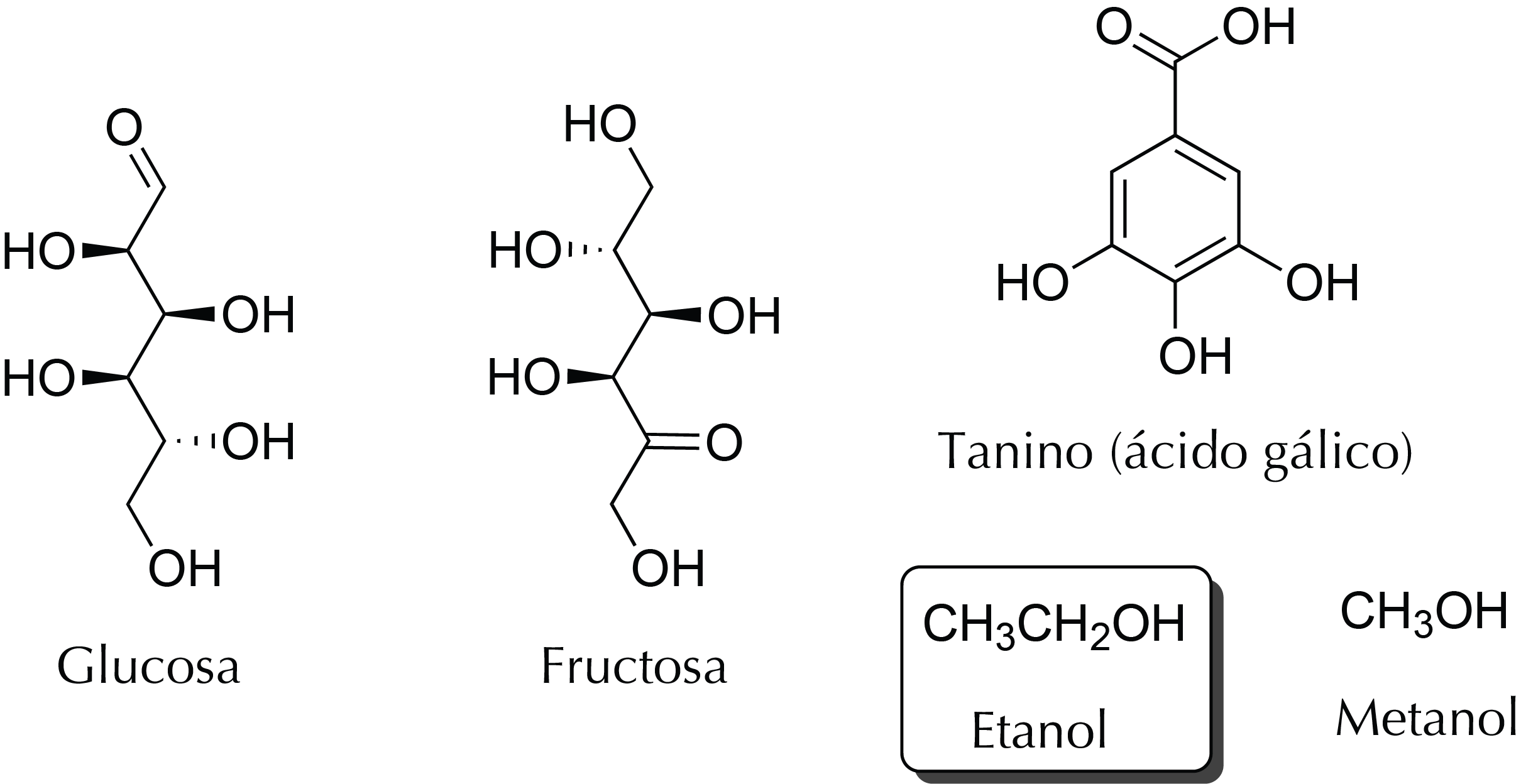
However, in the particular case of wine as it is a complex mixture containing water, EtOH, sugars, organic acids, pigments and other ingredients; therefore, the ABV cannot be determined directly from the density of the wine.
Therefore, a process is needed to separate EtOH from the rest of the components by simple distillation. The volatile components found in considerable quantities are water and EtOH, which have an e.p. of 100.0 and 78.3 ºC, respectively. Both can form azeotrope with an e.i.p. of 78.2 ºC and with a composition of 96 mass % EtOH (97 % v/v). In red wines, the ABV of EtOH is expressed in volume percent and is somewhat higher than 10 %.
In red wine distillation, no fraction containing 100 % EtOH can be produced because the most volatile component is the azeotrope. In this practice, no fraction will be produced; instead, all the EtOH contained in the sample will be distilled, in order to determine the total ABV content of the corresponding red wine.
Experimental procedure
With a graduated cylinder, measure 100 ml of wine and transfer it to a 250 ml round bottom flask. Wash the graduated cylinder with 50 ml of distilled water to remove any remaining liquid. Add the water to the contents of the flask.[1] Add a magnet and mount a simple distillation setup on the flask containing the wine sample and heat it with a magnetic stir plate, maintaining a homogeneous boiling.[2]
The distillate is collected in a Erlenmeyer flask, to which 10 ml of water is previously added so that the distillate does not evaporate, until reaching a volume of approximately 50 ml. The distillate is allowed to cool to room temperature and is made up to 100 ml (the same volume as the initial wine sample) in a graduated cylinder. With the help of a alcoholometer, determine the alcohol content of the wine.
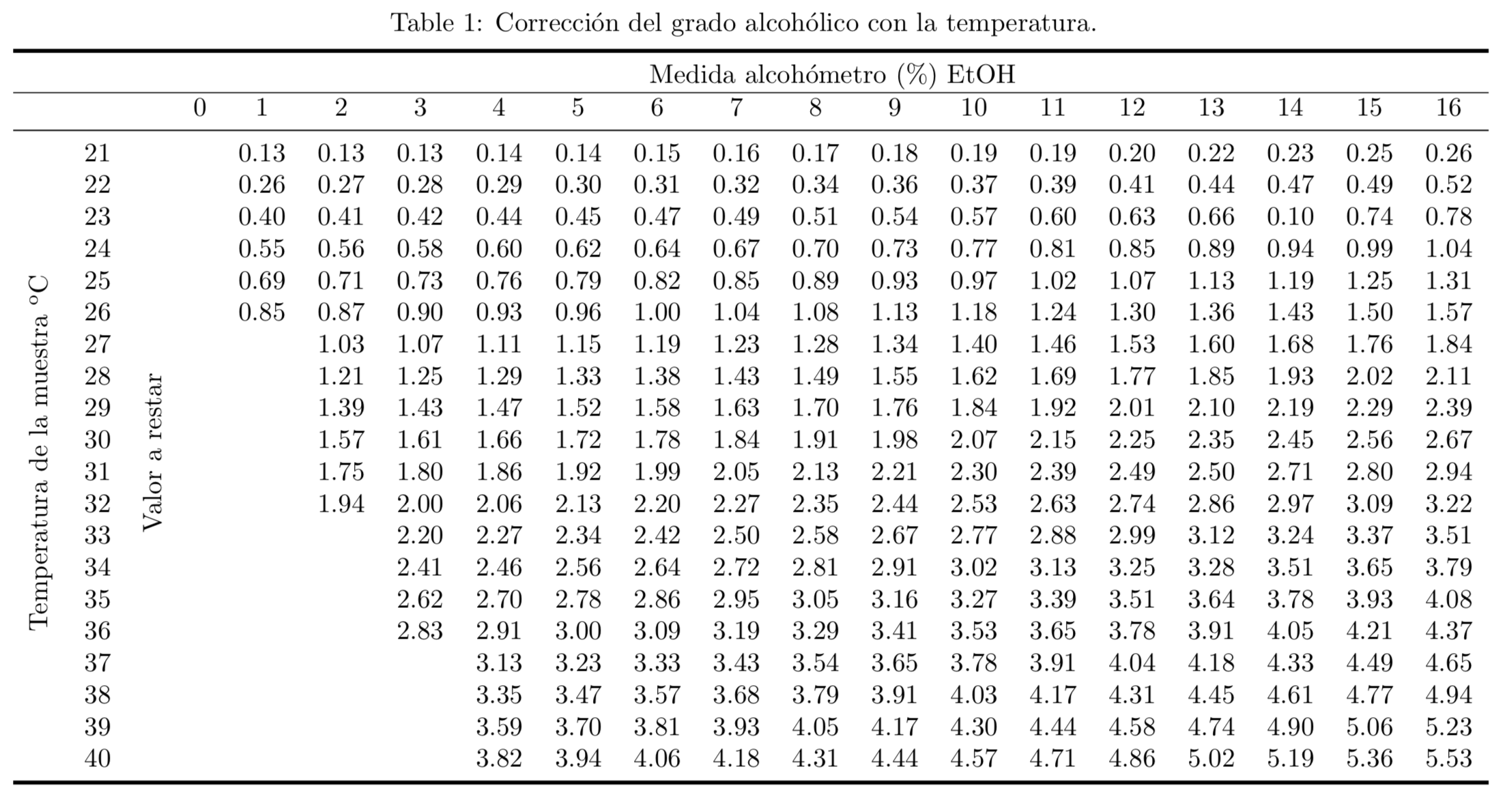
Since the density varies with temperature and the alcoholmeter is calibrated at 20 ºC, the temperature of the sample should be measured and the measurement corrected, if necessary, with the values in Table 1.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| EtOH | 46.07 | -114.1 | 78.5 | 0.790 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| EtOH |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| EtOH | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
References and notes
- [1] The addition of this extra water ensures that the flask does not dry out during the distillation process.
- [2] Do not forget to connect the cooler condenser to the water intakes and open the circuit.
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2