Written by J.A Dobado | Last Updated on April 22, 2024
Objective
The production of an alkene (cyclohexene) by the acid-catalyzed dehydration reaction of an alcohol (cyclohexanol).
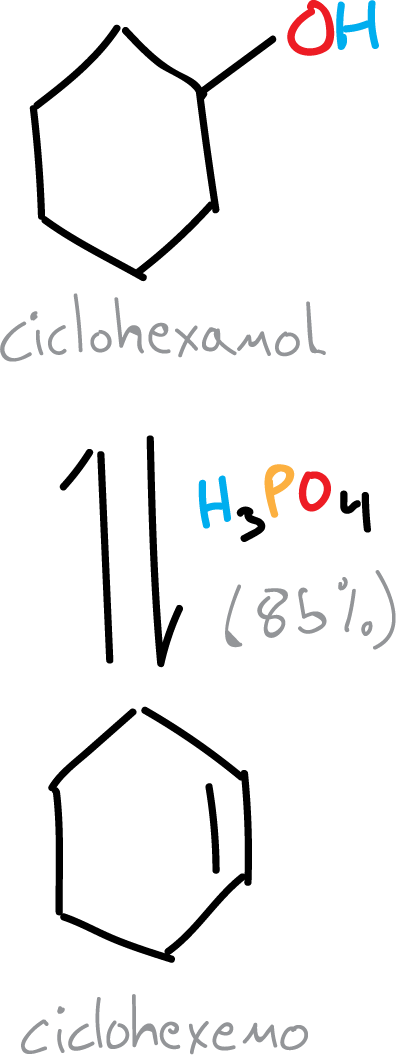
Background
A secondary alcohol, such as cyclohexanol, is dehydrated via an E1 mechanism, where the key intermediate is the cyclohexyl cation. This cation can react by either an elimination or a substitution reaction. To obtain the alkene in good yield, it is necessary to suppress the competitive substitution side reaction. In this experiment, the elimination reaction is favored by using strong acids (with anions that are relatively weak nucleophiles), high reaction temperatures and distillation of the cyclohexene as it is formed.
The acid-catalyzed dehydration reaction of an alcohol is a reversible unimolecular elimination reaction following the Saytzev rule (Saytzeff’s rule). As a dehydrating agent, any strong acid (sulfuric acid H2SO4, phosphoric acid H3PO4 or oxalic acid (COOH)2 can be used.
Traditionally, this reaction has been carried out by the sulfuric acid method, but the phosphoric acid method is described here, since it has certain advantages over the sulfuric acid method, such as no carbonization of the reagents and reduced risk of handling.
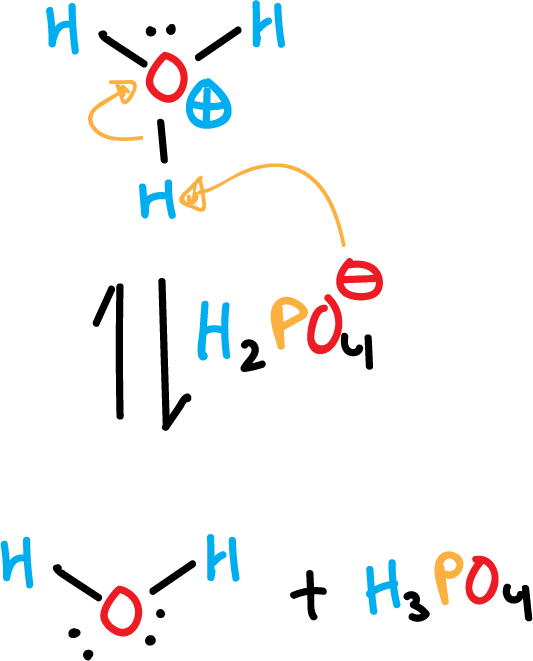
Reaction mechanism
In first place, the protonation of the hydroxyl by a proton of the phosphoric acid takes place. This first step occurs very quickly.
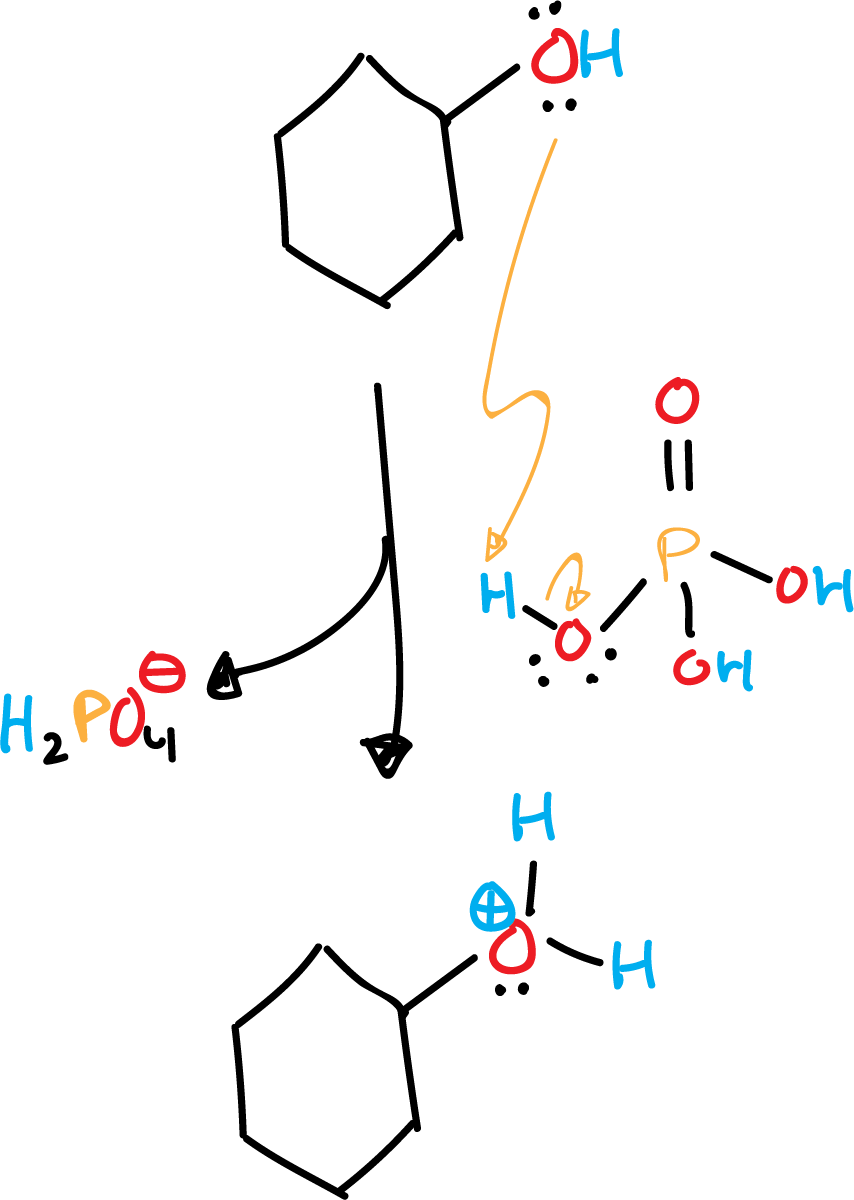
Subsequently, a second stage of carbocation formation occurs, as the very weakened C-O bond is broken. This second step is the limiting step of the reaction, and occurs slowly.
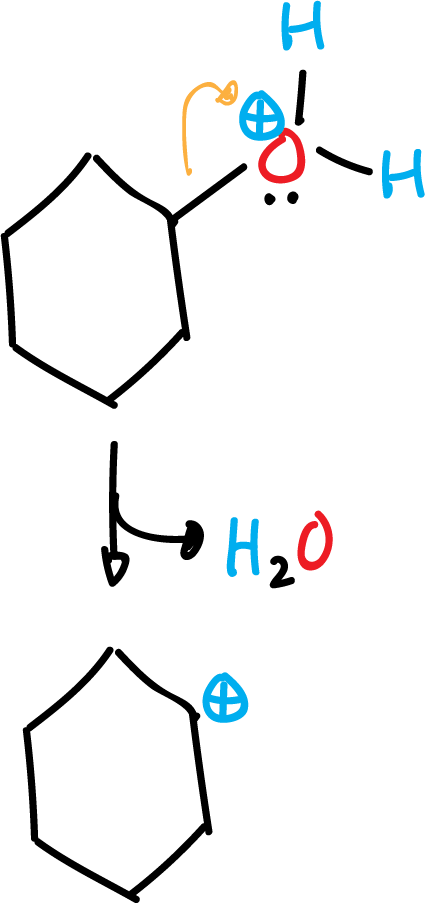
Once the carbocation has been formed, with the help of a water molecule an abstraction of a proton is produced. This is a fast process, where cyclohexene is obtained.
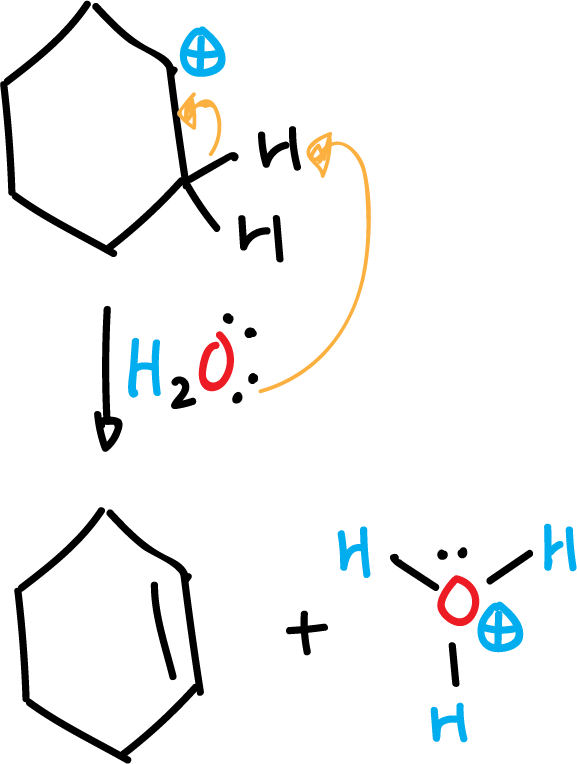
At the same time and also rapidly, the hydronium cation (H3O⊕) gives up a proton to phosphate, to give phosphoric acid and water.
Experimental procedure
Place 20 g (21 ml) of cyclohexanol and 5 ml of phosphoric acid (85 %) H3PO4 in a 100 ml round bottom flask. Prepare a simple distillation setup. Keep the 50 ml collecting flask immersed in an ice water bath, since the cyclohexene obtained is very volatile and flammable. The distillation should be carried out slowly.
Stop the distillation when approximately 8 ml appear in the distillation flask. Before discarding the distillation residue in the waste container, pour into a beaker with ice water. Transfer the distillate to a separatory funnel and add 10 ml of water, followed by 10 ml of saturated NaCl solution (brine). It is recommended to make the washes as fast as possible. Separate the organic layer together with cyclohexene from the separatory funnel. Dry in Erlenmeyer with 2 g of anhydrous calcium chloride (CaCl2), separating the desiccant from the cyclohexene by decantation. Due to the volatility of cyclohexene, filtration is not recommended in this case. Quickly collect the cyclohexene in a dry 50 ml round bottom flask and add 2 or 3 pieces of porous plates.
Perform a simple distillation and collect the fraction after distillation at 78-83 ºC. At the end of the distillation process the residue becomes yellowish/dark brown in color and the distillation temperature approaches 83 ºC. The collecting flask should be immersed in an ice/water bath. The estimated yield is 75 %.
| DANGER! “Perform distillation in fume hood.” |
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| H3PO4 | 98.00 | 40 | 158 | 1.685 |
| Cyclohexanol | 100.16 | 20-22 | 160-161 | 0.968 |
| Cyclohexene | 82.14 | -104 | 83 | 0.779 |
| CaCl2 | 110.98 | 782 | >1,600 | 2.15 |
| NaCl | 58.44 | 801 | 1,413 | 2.165 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| H3PO4 |  |
| Cyclohexanol |  |
| Cyclohexene |    |
| CaCl2 |  |
| NaCl | Non-hazardous |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| H3PO4 | NBIIXXVUZAFLBC-UHFFFAOYSA-N |
| Cyclohexanol | HPXRVTGHNJAIIH-UHFFFAOYSA-N |
| Cyclohexene | HGCIXCUEYOPUTN-UHFFFAOYSA-N |
| CaCl2 | UXVMQQNJUSDDNG-UHFFFAOYSA-L |
| NaCl | FAPWRFPIFSIZLT-UHFFFAOYSA-M |
Video on cyclohexanol dehydration
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Cyclohexanol dehydration: A simple experiment in heterogeneous catalysis
A. Costa
Journal of Chemical Education 1982 59 (12), 1066
DOI: 10.1021/ed059p1066