Written by J.A Dobado | Last Updated on April 22, 2024
Objective
The purpose of this experiment is to obtain an asymmetric ether of industrial interest from naphthalen-2-ol (β-naphthol) using two synthetic methods.
Background
Ethers can be obtained from alcohols by nucleophilic substitution reactions (SN2).
2-methoxynaphthalene (β-naphthyl methyl ether) is a white solid with a melting point of 73 °C and is known commercially as neroline. It is used in perfumery for its floral odour, as a stabiliser for speciality powders and as an intermediate for the synthesis of non-steroidal anti-inflammatory drugs. It can be obtained by two synthetic methods: using the Williamson reaction or by treatment of MeOH and β-naphthol (naphthalen-2-ol) with hot H2SO4.
As is well known, the Williamson ether synthesis is a process that allows the preparation of a wide range of ethers, both symmetrical and asymmetrical, as long as the alcohol is not hindered, since the structure of each reactant can be easily varied. The reaction proceeds from an alcohol and an alkyl halide in a basic medium.
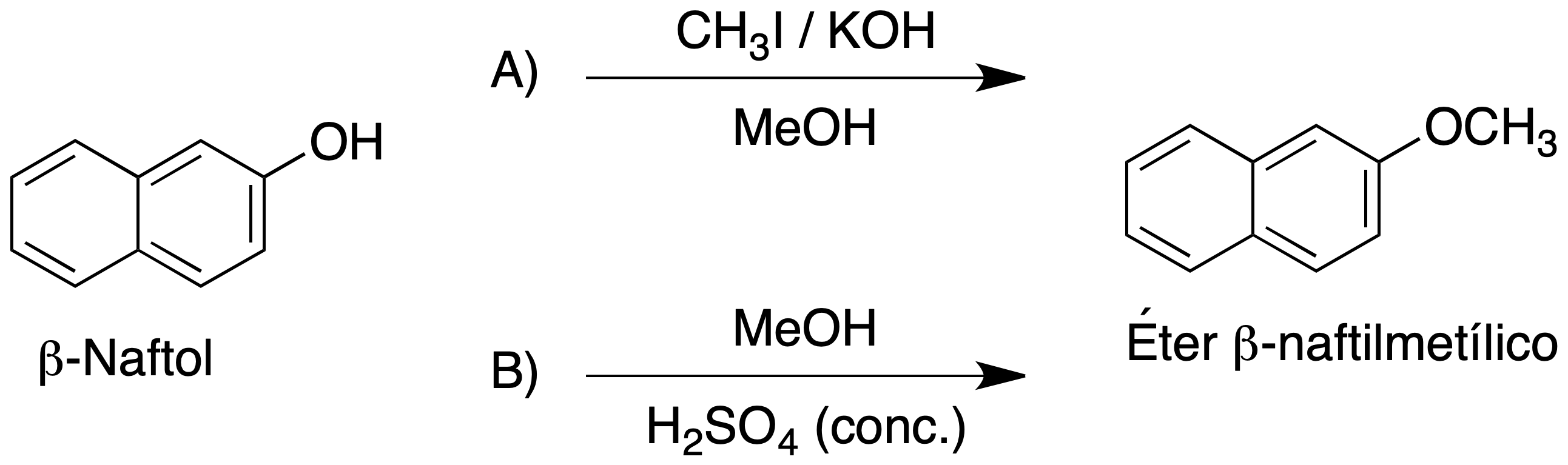
Ethers can also be prepared by reacting an alcohol in an acid medium. In this case, the hydroxyl (a very bad leaving group) is protonated into an excellent leaving group (alkyl oxonium ion), as water is released. This allows the reaction with not so good nucleophiles, such as another alcohol molecule, via a nucleophilic substitution reaction.
In this second procedure, which we will use in this experiment, the alkoxonium ion is formed by the reaction of MeOH with H2SO4. In a further step, the alkoxonium ion is attacked by a molecule of β-naphthol producing the corresponding β-naphthyl methyl ether.
Normally this second method allows only symmetrical ethers to be obtained. An additional problem is the tendency to dehydration to form alkenes, which is highly favoured with secondary alcohols.
Reaction mechanisms
Ether formation in basic medium
Ether formation takes place in basic media, the mechanism through which this process takes place involves the following steps:
- Deprotonation of β-naphthol in basic medium.
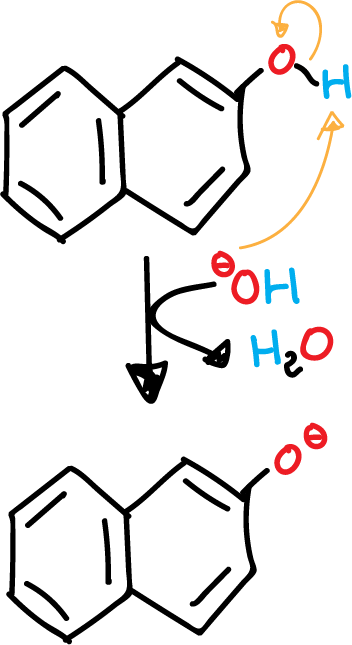
The β-naphthol has a phenol (aromatic alcohol) that can be easily deprotonated. A phenolate (aromatic alkoxide) is obtained, which is more nucleophilic than the initial alcohol.
- Nucleophilic substitution reaction.
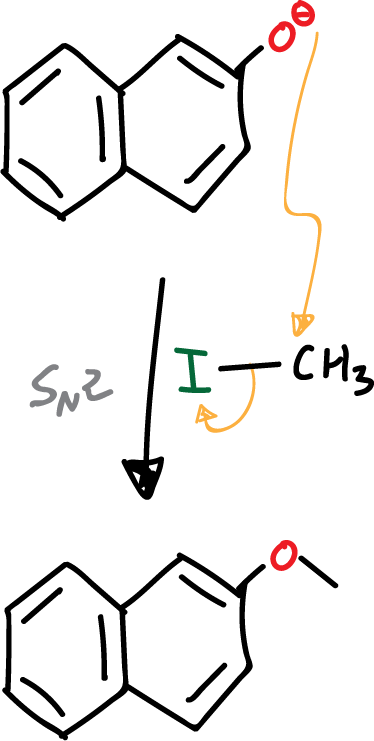
Subsequent to deprotonation, the nucleophilic phenolate is attacked by alkyl iodide. Since we have a good nucleophile and a very good leaving group on a primary carbon, the reaction proceeds via an SN2-type mechanism.
Ether formation in an acidic medium
- Protonation of methanol in acidic medium
Alcohols are amphoteric in character, acting as acids or bases. In this case, when an alcohol is treated with a strong acid, the alcohol acts as a base and is protonated.
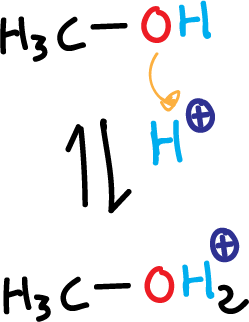
This transforms the hydroxyl (HO⊕), which is a bad leaving group, into an excellent leaving group (alkyl oxonium ion) since water, a neutral molecule, is released. They can therefore be involved in nucleophilic substitution reactions.
- Nucleophilic substitution reaction.
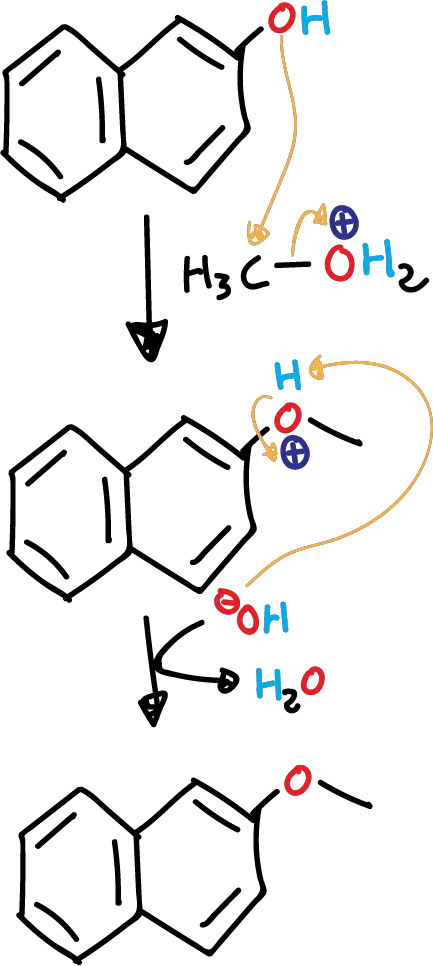
The protonated form of methanol reacts with naphthol to form the corresponding ether. Being a primary carbon atom, the reaction again proceeds via an S2-type mechanism.
It should be noted that, in this case, the ether synthesis in acidic media does not compete with elimination reactions since none of the alcohols can give elimination reactions.
The reaction of the methyloxonium ion with MeOH instead of 2-naphthol could occur, but in that case the product is dimethyl ether, which is very volatile (e.g. = -24 °C) and is removed from the medium. This fact makes it possible to obtain the non-symmetrical ether between 2-naphthol and methanol in an acidic medium.
Experimental procedure
Obtaining β-naphthyl methyl ether by Williamson synthesis
In a 100 ml round bottom flask fitted with a stir bar, 2.88 g (20 mmol) of β-naphthol (2-hydroxynaphthalene), 1.46 g (26 mmol) KOH and 20 ml of MeOH are mixed. A stopper is attached and the mixture is stirred at room temperature until dissolution of the solid. With the aid of a syringe, 1.4 ml (23 mmol) methyl iodide (CH3I) is added.
| DANGER! “Conduct the experiment in a fume cupboard due to the hazardous nature of methyl iodide (CH3I).” |
Next, a reflux condenser and a drying tube are coupled and heated for 1 h. The mixture is allowed to cool to room temperature and water is added until a precipitate appears. The mixture is cooled to room temperature and water is added until a precipitate appears. The solid is filtered under vacuum and recrystallized in EtOH (estimated yield 75 %, melting point 73 °C).
Obtaining β-naphthyl methyl ether in an acid medium
In a 100 ml round bottom flask, 5 g of β-naphthol, 25 ml of MeOH and 5 ml of H2SO4 are placed. The mixture is kept at reflux for 1 h.
The reaction crude is allowed to cool and poured over 100 ml ice/water. The precipitated ether is filtered off under vacuum. The precipitate is washed in the Büchner twice with ice water, once with 20 ml 10 % NaOH and once with ice water.
The product obtained is recrystallized in EtOH, decolorized with activated carbon if necessary (estimated yield 70 %).
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| β-Naphthol | 144.17 | 120-122 | 285-286 | 1.280 |
| CH3I | 141.94 | -64 | 41-43 | 2.280 |
| H2SO4 | 98.08 | 3 | - | 1.80-1.84 |
| EtOH | 46.07 | -114.1 | 78.5 | 0.790 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
| MeOH | 32.04 | -98 | 64.7 | 0.791 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| β-Naphthol |   |
| CH3I |   |
| H2SO4 |  |
| EtOH |  |
| NaOH |  |
| MeOH |    |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| β-Naphthol | JWAZRIHNYRIHIV-UHFFFAOYSA-N |
| CH3I | INQOMBQAUSQDDS-UHFFFAOYSA-N |
| H2SO4 | QAOWNCQODCNURD-UHFFFAOYSA-N |
| EtOH | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
| MeOH | OKKJLVBELUTLKV-UHFFFAOYSA-N |
Video on obtaining β-naphthyl methyl ether
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- A Multioutcome Experiment for the Williamson Ether Synthesis
Kasey L. Yearty, Ryan K. Maynard, Christina N. Cortes, and Richard W. Morrison (2020) “A Multioutcome Experiment for the Williamson Ether Synthesis” Journal of Chemical Education, 97 (2), 578-581
DOI: 10.1021/acs.jchemed.9b00503 - Vogel, A.I., Furniss, B.S., Hannaford, A.J., Tatchell, A.R., and Smith, P.W.G. (1989). Vogel’s Textbook of Practical Organic Chemistry (Vogel’s Textbook series). Longman. ISBN: 9780470214145