Written by J.A Dobado | Last Updated on April 22, 2024
Objective
To learn how to set up Soxhlet extraction to obtain oil from sunflower seeds.
Background
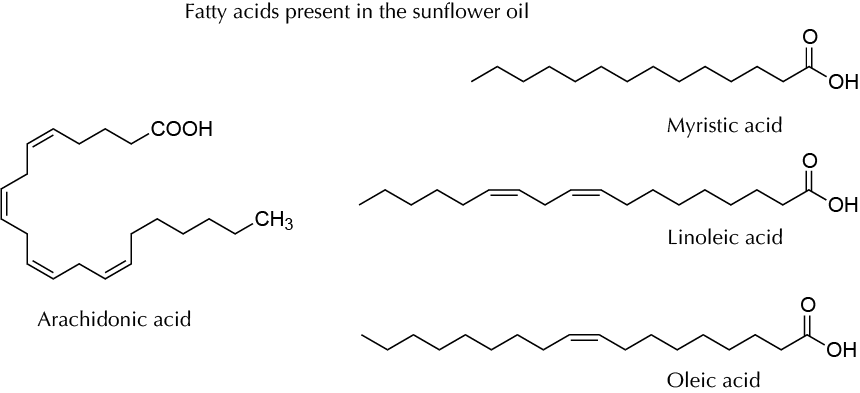
Vegetable oils are mainly triglycerides from seeds or fruits. They are a key component of the human diet and are also used for other purposes, including the production of biofuels (biodiesel), lubricants, and cosmetics such as soaps and creams. They are often produced by mechanical or chemical processes or a combination of the two. One of the most common procedures to produce vegetable oils is extraction from the oil-rich parts of the plant using organic solvents such as hexane. In the case of sunflower seed, the oil content can be as high as 40% of the dry weight of seeds. In this experiment, oil from sunflower seeds is obtained by means of continuous extraction (Soxhlet equipment) with hexane.
Procedure
Weigh 20 g of sunflower seeds. Using a mortar, grind seeds to form a material as homogeneous as possible. Prepare a piece of cartridge filter paper, and place the crushed seeds inside. Then fill the Soxhlet flask with 250 ml of hexane and add a stir bar. Place the cartridge with the crushed seeds in the extractor. Set up the system and keep it running for two or three extraction cycles, overall approximately 30 min.
Disassemble the Soxhlet and add 120 ml of warm water to the flask. Then, connect the flask to a distillation adapter (three-way adapter) with a thermometer, water-jacketed condenser, distilling adapter, and receiving flask. Continue with the steam distillation (internal source of vapor) until only distilled water remains to ensure that the hexane has been completely removed from the oil.
Allow the flask content and the receiving content to cool to r.t. and then pour independently into a separatory funnel for separation. The distillate will be composed of a mixture of hexane (upper phase) and water, and separation of the two phases allows recovery of the organic solvent (hexane) for reuse. Then the residue remaining after distillation will consist of a mixture of water and oil (upper phase). Once the oil is separated, measure the volume in a graduated cylinder, previously tared, weigh, and calculate the yield and the approximate density of the oil.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Arachidonic acid | 304.47 | -49 | 169-171 | 0.922 |
| Hexane | 86.18 | -95 | 69 | 0.659 |
| Linoleic acid | 280.45 | -5 | 229-230 | - |
| Myristic acid | 228.37 | 52-54 | 250 | - |
| Oleic acid | 282.46 | 13-14 | 194-195 | 0.890 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Arachidonic acid | Non-hazardous |
| Hexane |     |
| Linoleic acid | Non-hazardous |
| Myristic acid |  |
| Oleic acid |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Arachidonic acid | YZXBAPSDXZZRGB-DOFZRALJSA-N |
| Hexane | VLKZOEOYAKHREP-UHFFFAOYSA-N |
| Linoleic acid | OYHQOLUKZRVURQ-HZJYTTRNSA-N |
| Myristic acid | TUNFSRHWOTWDNC-UHFFFAOYSA-N |
| Oleic acid | ZQPPMHVWECSIRJ-KTKRTIGZSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- M. D. Luque de Castro and L. E. García-Ayuso, Soxhlet extraction of solid materials: an outdated technique with a promising innovative future, Analytica Chimica Acta 369 (1998), no. 1-2, 1–10, DOI: 10.1016/S0003-2670(98)00233-5