Objetive
To prepare a cyclic amide from p-chloroaniline and maleic anhydride.
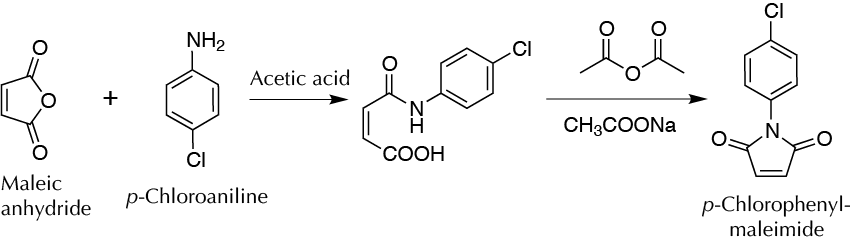
Background
Obtaining N-(p-chlorophenyl)-maleimide from the p-chloroaniline and maleic anhydride is a two-step process. First, a nucleophilic attack of the nitrogen (aromatic amine), catalyzed by the acid medium on the carboxylic carbon of the cyclic anhydride, occurs. In the second step, the N-(p-chlorophenyl)-maleamic acid is heated, and nitrogen attacks the other carboxylic carbon to form the N -(p-chlorophenyl)-maleimide.
Experimental procedure
A) Formation of N-(p-chlorophenyl)-maleamic acid:
In a 100 ml round-bottom flask, pour 3.8 ml of p-chloroaniline and 6 ml of acetic acid. Heat the mixture slightly with magnetic stirring until complete dissolution of the solid. Then slowly add 2.94 g of maleic anhydride while stirring until a precipitate appears. Isolate the resulting solid by vacuum filtration and wash with acetic acid (3 ml). Purify the product in this step by recrystallization from EtOH. Weigh the solid and calculate the yield for this step.
B) Formation of N-(p-chlorophenyl)-maleimide:
In a 100 ml round-bottom flask, place 3.3 g of N -(p-chlorophenyl)-maleamic acid, 6 ml of acetic anhydride, and 0.15 g of sodium acetate. Adjust quantities to the mass obtained in the previous stage.
Attach a reflux condenser to the flask and heat the mixture (water bath), at a temperature of 85–95 ºC, for 1 h with magnetic stirring. Remove the condenser and allow the flask to cool to r.t. Then insert into an ice bath and shake the flask occasionally until the appearance of a precipitate. Filter the maleimide formed by vacuum, and recrystallize from EtOH, using activated carbon to remove colored impurities. Dry the solid and then weigh and cal- culate yields (at this stage and the overall reaction).
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Acetic acid | 60.05 | 16.2 | 118 | 1.049 |
| Acetic anhydride | 102.09 | -73.1 | 139.8 | 1.080 |
| EtOH | 46.07 | -114.1 | 78.5 | 0.790 |
| Maleic anhydride | 98.06 | 51-56 | 200 | 1.480 |
| N-(4-Chlorophenyl)-maleamic acid | 225.019 | 176.28 | 466.16 | 1.449 |
| N-(4-Chlorophenyl)-maleimide | 207.61 | 110-112 | 350.63 | 1.46 |
| p-Chloroaniline | 127.57 | 72.5 | 232 | 1.140 |
| Sodium acetate | 82.03 | 328 | - | 1.528 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Acetic acid |   |
| Acetic anhydride |    |
| EtOH |  |
| Maleic anhydride |    |
| N-(4-Chlorophenyl)-maleamic acid | See MSDS |
| N-(4-Chlorophenyl)-maleimide | See MSDS |
| p-Chloroaniline |    |
| Sodium acetate | Non-hazardous |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Acetic acid | QTBSBXVTEAMEQO-UHFFFAOYSA-N |
| Acetic anhydride | WFDIJRYMOXRFFG-UHFFFAOYSA-N |
| EtOH | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
| Maleic anhydride | FPYJFEHAWHCUMM-UHFFFAOYSA-N |
| N-(4-Chlorophenyl)-maleamic acid | FBTQVXSNFILAQY-WAYWQWQTSA-N |
| N-(4-Chlorophenyl)-maleimide | FPZQYYXSOJSITC-UHFFFAOYSA-N |
| p-Chloroaniline | QSNSCYSYFYORTR-UHFFFAOYSA-N |
| Sodium acetate | VMHLLURERBWHNL-UHFFFAOYSA-M |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- M. P. Cava, A. A. Deana, K. Muth, and M. J. Mitchell, N-Phenylmaleimide, Organic Synthesis; 41 (1961), 93. DOI: 10.15227/orgsyn.041.0093