Written by J.A Dobado | Last Updated on April 22, 2024
Objective
To form the C−C bond via condensation reaction of benzaldehyde catalyzed by cyanide ion or thiamine, and to perform the stereoselective reduction of benzoin.
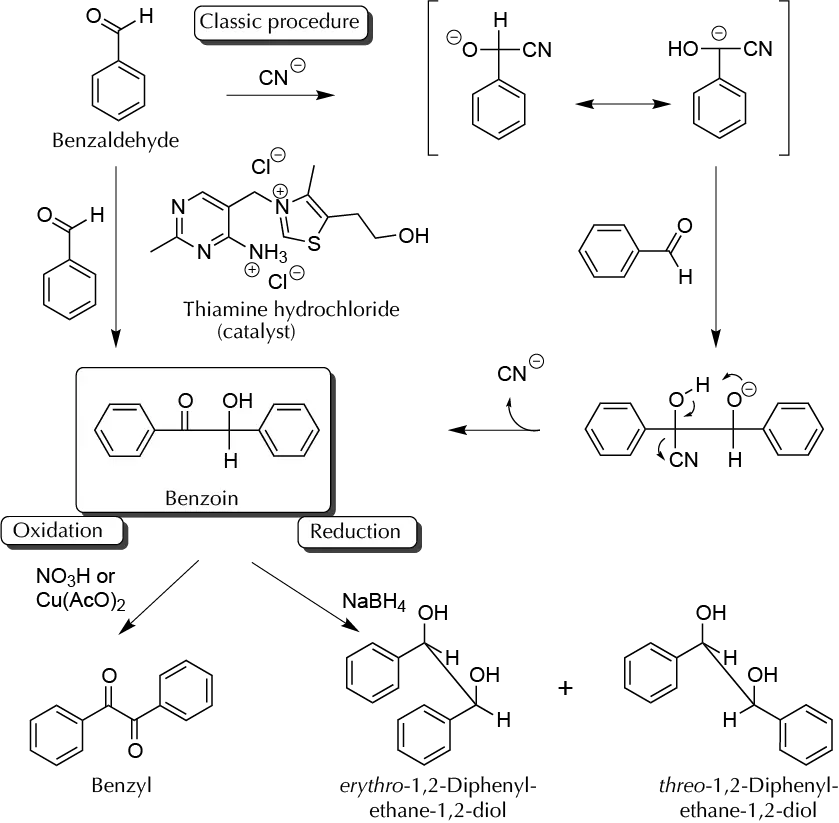
Background
One of the most important processes in Organic Synthesis is the formation of carbon-carbon bonds. Usually, this reaction requires the formation of a carbanion (carbon nucleophile) and its reaction with an electrophilic carbon (electron deficient).
The benzoin condensation involves the reaction of two molecules of benzaldehyde (or another non-enolizable aldehyde). As in this case, the carbonyl group has a partial positive charge needed to invert the polarity in one of these. To achieve this, normally, a catalytic amount of cyanide ion is often used, resulting in the formation of a carbanion, which can attack another carbonyl group, yielding the C−C bond. Subsequent HCN loss occurs with the formation of benzoin.
Recently, it has been shown that thiamine (its hydrochloride, vitamin B1) is a very efficient catalyst for producing the benzoin condensation. Benzoin allows an easy synthesis of glycol by reduction of the carbonyl group. This reduction could give a mixture of two diols, with treo and erythro configurations. However, the reduction with NaBH4 produces mainly the isomer erythro (form meso), which can be determined by comparing the m.p. of the product compared to the pure isomers: eritro (m.p. 137 ºC) and treo (m.p. 119 ºC).
Procedure
A) Synthesis of benzoin:
Dissolve in a flask 0.7 g of thiamine hydrochloride (vitamin B1) in 1.5 ml of water. Then add 6–7 ml of EtOH 95%, and cool the solution in an ice bath. To this solution under stirring, add slowly (for 7 to 10 min) 1.5 ml of a cold solution of NaOH 3 M. Add 4.24 g (4 ml) of benzaldehyde, shake well, and check the pH. If the pH is less than 8.0, add slightly more of NaOH, to a pH value in the 8.0–9.0 range.
Be very careful on this point because if the pH is higher than 9.0, the Cannizzaro reaction occurs and, if the pH is too low, thiamine is not ionized.
Heat the solution in a water bath at 60–70 ºC for 1 h. Remove the bath and allow the reaction to reach r.t. Cool in an ice bath whereupon a precipitate should appear. Filter the reaction crude, and wash with 10 ml of cold water. An oil may also appear, this being a mixture of benzaldehyde and the reaction byproducts. Recrystallize the product from EtOH/water (m.p. 137 ºC).
B) Reduction of benzoin:
In a 100 ml Erlenmeyer, add 2 g of benzoin and 20 ml of absolute EtOH. Shake, and while shaking, add in small portions 400 mg of NaBH4. When the addition is finished, stir for another 15 min at r.t. Cool in an ice bath and decompose the NaBH4 excess, adding first 30 ml of water and then adding dropwise 1 ml of HCl 6 M. A foam could form if the acid is not added slowly enough. Add another 10 ml of water and stir for another 15 min. A white precipitate will appear. Filter the reaction product, and wash it with water. Air dry and measure the m.p. If the m.p. range is too broad, recrystallize
from acetone/hexane.
C) Benzoin oxidation:
In a round-bottom flask provided with a reflux condenser, place 2 g of benzoin, 3.75 g of cupric acetate monohydrate, 15 ml of acetic anhydride, and 5 ml of water. Heat by reflux for 15 min. Filter the hot mixture to remove the cuprous oxide. Cool the filtrate, and filter the precipitate that appears under vacuum, wash with a small amount of cold water, and air dry (m.p. 95 ºC).
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Acetic anhydride | 102.09 | -73.1 | 139.8 | 1.080 |
| Benzaldehyde | 106.12 | -26 | 178-179 | 1.044 |
| Benzoin | 212.24 | 134-138 | 194 | - |
| Copper(II) acetate monohydrate | 199.65 | 115 | - | 1.882 |
| Cu2O | 143.09 | 1,230 | - | - |
| EtOH | 46.07 | -114.1 | 78.5 | 0.790 |
| NaBH4 | 37.8 | 400 | - | 1.07 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
| Thiamine hydrochloride | 337.27 | 250 | - | - |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Acetic anhydride |    |
| Benzaldehyde |  |
| Benzoin | Non-hazardous |
| Copper(II) acetate monohydrate |   |
| Cu2O |   |
| EtOH |  |
| NaBH4 |    |
| NaOH |  |
| Thiamine hydrochloride | Non-hazardous |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Acetic anhydride | WFDIJRYMOXRFFG-UHFFFAOYSA-N |
| Benzaldehyde | HUMNYLRZRPPJDN-UHFFFAOYSA-N |
| Benzoin | UHOVQNZJYSORNB-UHFFFAOYSA-N |
| Copper(II) acetate monohydrate | NWFNSTOSIVLCJA-UHFFFAOYSA-L |
| Cu2O | BERDEBHAJNAUOM-UHFFFAOYSA-N |
| EtOH | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
| NaBH4 | YOQDYZUWIQVZSF-UHFFFAOYSA-N |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
| Thiamine hydrochloride | DPJRMOMPQZCRJU-UHFFFAOYSA-M |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Vogel, A.I., Furniss, B.S., Hannaford, A.J., Tatchell, A.R., and Smith, P.W.G. (1989). Vogel’s Textbook of Practical Organic Chemistry (Vogel’s Textbook series). Longman. ISBN: 9780470214145
- A. T. Rowland, The determination of the stereochemistry of erythro-1,2-diphenyl-1,2- ethanediol: an undergraduate organic experiment, Journal of Chemical Education 60 (1983), no. 12, 1084, DOI: 10.1021/ed060p1084