Written by J.A Dobado | Last Updated on April 22, 2024
Objective
To produce biodegradable polymers from renewable raw materials with different mechanical properties by the method of preparation.
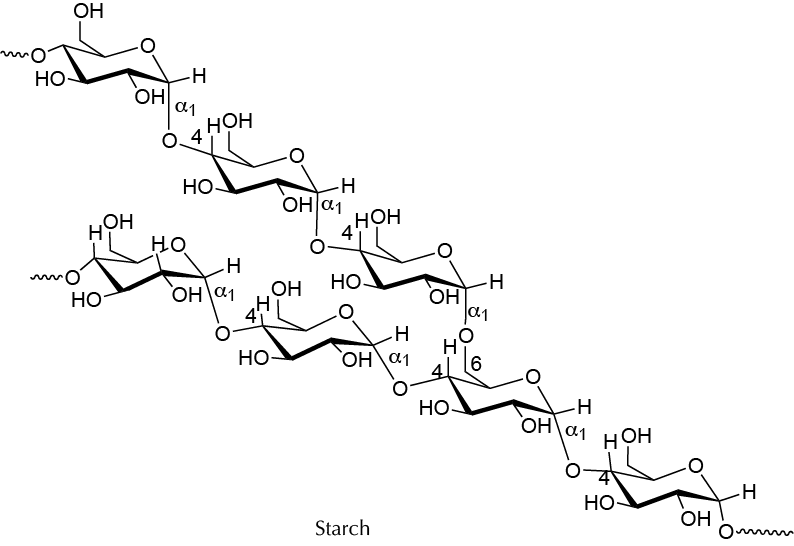
Background
Starch is a natural polymer present in cereals or potatoes, consisting of glucose units. It is a fundamental component of the human diet, but in turn can be used as a basis for the development of semisynthetic polymers with multiple applications, by adding molecules such as urea or glycerol that are inserted in the chains of amylose and/or amylopectin, modifying mechanical properties of the natural polymer. Based on these properties, different polymers will be prepared in this experiment.
Experimental procedure
Potato starch can be extracted by grating 100 g of potatoes using a kitchen grater. Place the grated potato in a mortar, add 100 ml of water, and crush using the pestle until the grated potato forms a paste as homogeneous as possible. Filter the mixture through a colander over a beaker. The operation is performed twice on each potato sample to increase efficiency in the production of starch. The mixture is allowed to stand until it forms a paste deposit in the beaker. Decant to remove most of the supernatant with the aid of a Pasteur pipette, avoiding stirring. Remove the water that could not be removed previously by decantation, and, on this material, perform the following operation twice to provide two samples of starch.
A) Preparation of a brittle polymer
To a starch sample obtained according to the above procedure, add 20 ml of water and 3 ml of HCl (0.1 M). Gently heat the mixture for 15 min, trying not to bring it to a boil. Dropwise, add a solution of NaOH (0.1 M) until neutral, checking the result with a piece of indicator paper. The result is a viscous liquid, to which 2 drops of food coloring (tartrazine), are added. Mix using a glass rod to form a smooth paste. Spread on a watch glass to a film and place in an oven at 60 ºC for approximately 1.5 h, or allow to air dry overnight.
B) Preparation of a flexible polymer
A similar procedure to the above-mentioned case is followed but adding 2 ml of glycerol as a plasticizer.
Compare the properties of the materials produced by the two different methods.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Glycerol | 92.09 | 20 | 182 | - |
| HCl | 36.46 | -30 | >100 | 1.200 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
| Potato starch | - | - | - | 1.500 |
| Tartrazine | 534.36 | - | - | - |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Glycerol | Non-hazardous |
| HCl |   |
| NaOH |  |
| Potato starch | Non-hazardous |
| Tartrazine |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Glycerol | PEDCQBHIVMGVHV-UHFFFAOYSA-N |
| HCl | VEXZGXHMUGYJMC-UHFFFAOYSA-N |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
| Potato starch | |
| Tartrazine | RHKOFJVEQBGAMP-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2