Written by J.A Dobado | Last Updated on April 22, 2024
Objective
To produce a slime, biodegradable polymer resulting from the reaction of polyvinyl alcohol (PVA) and borax.
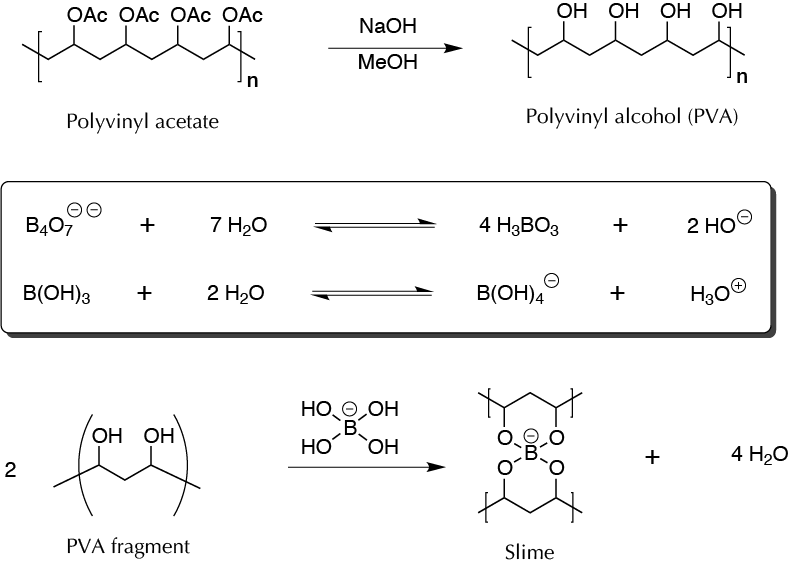
Background
This name is applied to a non-toxic and biodegradable polymer prepared from polyvinyl alcohol (PVA), and borax, food coloring (tartrazine), or fluorescein can be added to the polymer. PVA is a polymer that in turn is synthesized from polyvinyl acetate by treating this with NaOH in MeOH. This is not a polymerization process, but rather the transformation of one polymer into another. Depending on the type of PVA used, it is a more or less dense material that, according to its characteristics, is used for the manufacture of toys, fluid puncture repair kits, or special effects in the entertainment industry for viscous blood and masks to characterize zombies and corpses, simulate wounds, etc. Borax is the sodium tetraborate decahydrate (Na2B4O7 · 10H2O) that, when dissolved in water, is hydrolyzed to boric acid and HO– anions, yielding a pH of about 9.13. Subsequently, the boric acid reacts with water to form the borate anion. When an aqueous solution of borax and PVA is mixed, a borate ion is sandwiched between the polymer chains, forming covalent bonds, and leading to a change in its properties to a viscous material.
Experimental procedure
Dissolve 1 g of borax in 25 ml of water. Simultaneously, prepare another solution of 4 g of PVA in 100 ml of water. To achieve complete dissolution, it is convenient to gently warm the mixture at a temperature not exceeding 50 ºC.
At this point, add a few drops of food coloring (tartrazine) or fluorescein to the dissolution of alcohol, and mix both solutions. A slime forms quickly and can be stored in a plastic bag with self-closing mechanism to prevent drying. In the case of the addition of fluorescein, the fluorescence of the material can be checked with a UV lamp, illuminating a prepared sample.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Borax | 201.22 | 741 | - | 2.367 |
| Fluorescein | 332.31 | 320 | - | - |
| MeOH | 32.04 | -98 | 64.7 | 0.791 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
| Polyvinyl alcohol | - | 200 | - | 1.269 |
| Tartrazine | 534.36 | - | - | - |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Borax |  |
| Fluorescein |  |
| MeOH |    |
| NaOH |  |
| Polyvinyl alcohol | Non-hazardous |
| Tartrazine |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Borax | CDMADVZSLOHIFP-UHFFFAOYSA-N |
| Fluorescein | GNBHRKFJIUUOQI-UHFFFAOYSA-N |
| MeOH | OKKJLVBELUTLKV-UHFFFAOYSA-N |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
| Polyvinyl alcohol | |
| Tartrazine | RHKOFJVEQBGAMP-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2