Written by J.A Dobado | Last Updated on April 22, 2024
Objetive
To acquaint students with the concept of chiral resolution: separation of the two enantiomers of a chiral compound. In this case, the different solubility of two diastereomeric salts in water will be used.
Background
When products, reagents, or non-chiral catalysts are used in a chemical reaction in which there are chiral centers, the overall result is the same as producing an optically inactive mixture of stereoisomers. If a chiral carbon or center is generated, the product will always appear as a racemic mixture (50% of the two possible enantiomers).
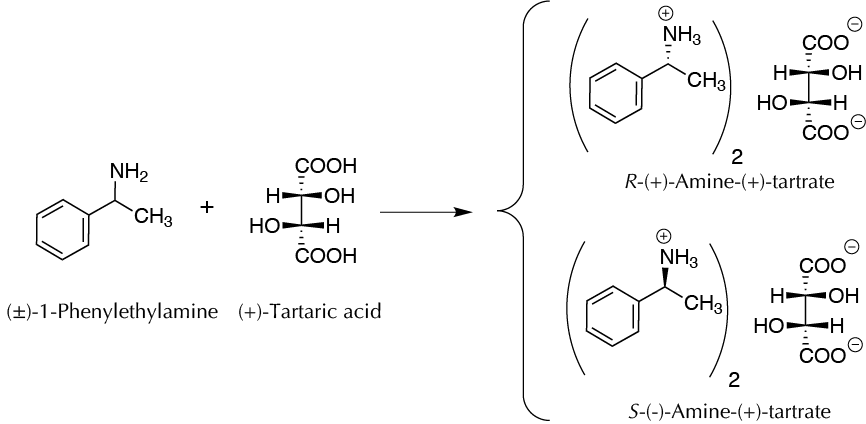
For the separation of the two enantiomers, a process called chiral resolution is carried out, reacting the racemic mixture with an optically active compound (chiral resolving agent). This results in the formation of two diastereomers, which possess different physical and chemical properties and therefore are capable of being separated by different procedures.
After the α-phenylethylamine racemic is synthesized (or purchased), the next procedure is to produce one of the optically pure enantiomers. In practice, only one of the two enantiomers separated is optically pure, the levorotatory, because it can be isolated more easily than can the other enantiomer. The chiral resolving agent to be used is (+)-tartaric acid, which forms two diastereomeric salts with α-methylbenzylamine (α-phenylethylamine) racemic.
The optically pure (+)-tartaric acid is very abundant in nature and is yielded mainly as a byproduct of winemaking. The (–)-amine-(+)-tartrate salt is less soluble than the salt (+)-amine-(+)-tartrate salt and precipitates from solutions in crystalline form. The crystals are filtered and purified. The salt is then treated with a diluted base, which is isolated with the free (–)-amine. On the other hand, the mother liquor, which preferably contains (+)-amine-(–)-tartrate salt, may be purified to produce the other diastereomeric salt. The hydrolysis of this salt would give the (+)-amine.
Experimental Procedure
Add 6.25 g of L-(+)-tartaric acid with 90 ml of MeOH to a 250 ml Erlenmeyer flask. Heat the mixture in a water bath until almost boiling. Add 5 g of racemic α-methylbenzylamine slowly because the mixture generates foam and can spill. Close the flask with a septum, and cool slowly for a day for good crystallization of the (+)-amine-(-)-tartrate salt.
In case of the formation of needle-shaped crystals, they can be redissolved by gentle heating and re-cooled. By this crystallization method, crystals of the desired shape can be produced. Note that the needle-shaped crystals do not have a sufficiently high optical purity to then achieve good resolution of the enantiomers.
Filter the crystals under a vacuum and wash with a few milliliters of cold MeOH. A second amount of crystals can be obtained by concentrating the mother liquor, which will give an additional amount of approximately 1.5 g of the salt. Once the sufficient amount of crystals has been produced, partially dissolve 10 g salt in 40 ml of water (If the procedure fails to provide 10 g of salt, reagent amounts should be adjusted to the mass of salt obtained). Add 6 ml of NaOH 50% solution, and extract the mixture with diethyl ether (2 × 20 ml).
Dry the organic layer (upper) on Na2SO4 (anhydrous). Then remove the desiccant by gravity filtration. The solvent is removed in a rotary evaporator to give a residue that is purified by vacuum distillation. The estimated yield is 55 %, and b.p. is 184–186 ºC.
Measure the optical rotation of the product. For this, accurately weigh 20 mg of amine and dissolve in 2 ml MeOH (measured by pipette). Calculate the total volume of the solution considering the sum of the volumes of the components (the amine density is 0.9395 g/ml).
Transfer the solution to the polarimeter tube and determine the optical rotation ([α]D20 = −40.3 ºC). Calculate the specific optical rotation and optical purity of the solution.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Acetic acid | 60.05 | 16.2 | 118 | 1.049 |
| Acetic anhydride | 102.09 | -73.1 | 139.8 | 1.080 |
| EtOH | 46.07 | -114.1 | 78.5 | 0.790 |
| Maleic anhydride | 98.06 | 51-56 | 200 | 1.480 |
| N-(4-Chlorophenyl)-maleamic acid | 225.019 | 176.28 | 466.16 | 1.449 |
| N-(4-Chlorophenyl)-maleimide | 207.61 | 110-112 | 350.63 | 1.46 |
| p-Chloroaniline | 127.57 | 72.5 | 232 | 1.140 |
| Sodium acetate | 82.03 | 328 | - | 1.528 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Acetic acid |   |
| Acetic anhydride |    |
| EtOH |  |
| Maleic anhydride |    |
| N-(4-Chlorophenyl)-maleamic acid | See MSDS |
| N-(4-Chlorophenyl)-maleimide | See MSDS |
| p-Chloroaniline |    |
| Sodium acetate | Non-hazardous |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Acetic acid | QTBSBXVTEAMEQO-UHFFFAOYSA-N |
| Acetic anhydride | WFDIJRYMOXRFFG-UHFFFAOYSA-N |
| EtOH | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
| Maleic anhydride | FPYJFEHAWHCUMM-UHFFFAOYSA-N |
| N-(4-Chlorophenyl)-maleamic acid | FBTQVXSNFILAQY-WAYWQWQTSA-N |
| N-(4-Chlorophenyl)-maleimide | FPZQYYXSOJSITC-UHFFFAOYSA-N |
| p-Chloroaniline | QSNSCYSYFYORTR-UHFFFAOYSA-N |
| Sodium acetate | VMHLLURERBWHNL-UHFFFAOYSA-M |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Vogel, A.I., Furniss, B.S., Hannaford, A.J., Tatchell, A.R., and Smith, P.W.G. (1989). Vogel’s Textbook of Practical Organic Chemistry (Vogel’s Textbook series). Longman. ISBN: 9780470214145
- A. Ault, Resolution of D,L-α-phenylethylamine: an introductory organic chemistry experiment, Journal of Chemical Education 42 (1965), no. 5, 269, DOI: 10.1021/ed042p269