Written by J.A Dobado | Last Updated on April 22, 2024
Objective
To perform a Diels-Alder reaction using water as a solvent, instead of using high aromatic boiling point solvents as commonly used in organic reactions.
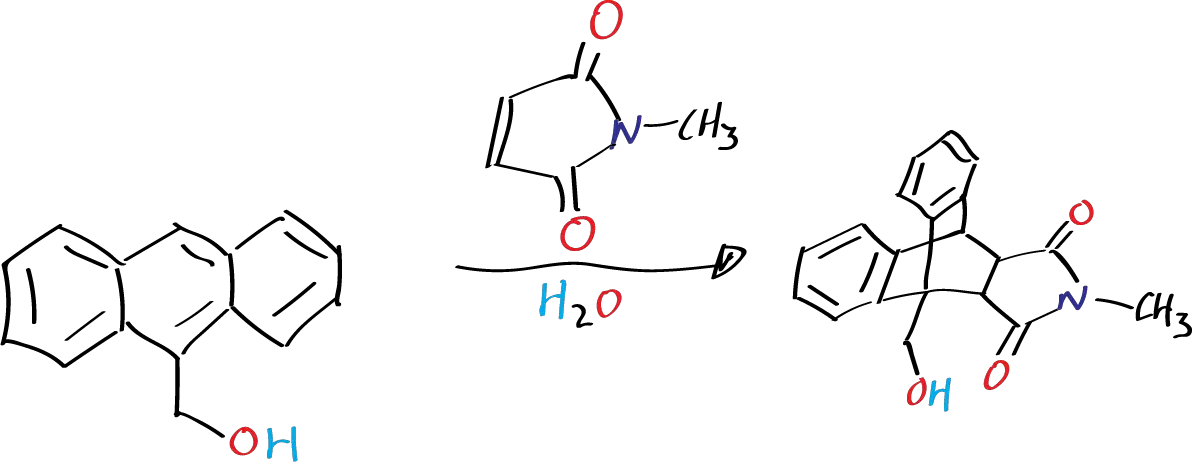
Background
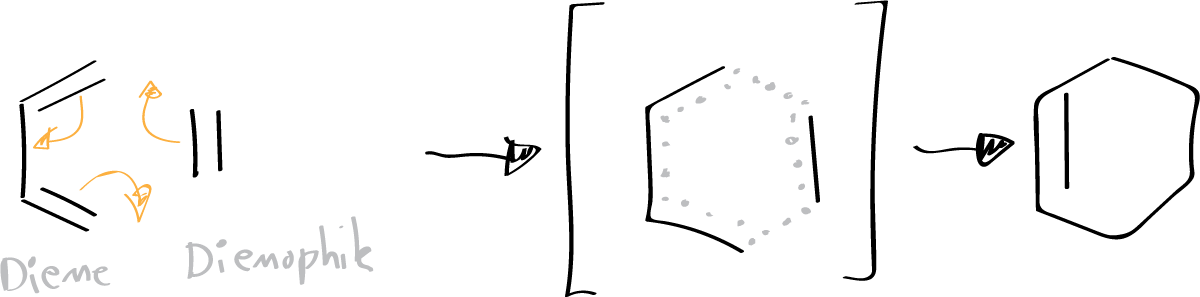
Diels-Alder reactions are [4+2] cycloadditions that allow the formation of six-membered ring systems from a diene and a dienophile.
They are considered some of the most notable synthetic processes for the formation of C−C bonds, and because of their effectiveness, relatively little energy is required for its implementation, enabling high atom economy. Many Diels-Alder reactions can be performed in water, not only because of the environmental benefits presented by the use of this universal solvent but because a significant acceleration often occurs in the reaction rate of such transformations due to the hydrophobic effects that occur in the water. In the following experiment, the Diels-Alder reaction is carried out between 9-anthracenemethanol and N-methylmaleimide.
Experimental procedure
Place 100 mg of 9-anthracenemethanol and 50 ml of water in a 100 ml round-bottom flask with magnetic stirring. Add 160 mg of N-methylmaleimide, and then attach a water condenser and heat the reaction to reflux for approximately 1 h. The reaction course is monitored by TLC, using a mixture of ethyl ac- etate/hexane (1:1) as an eluent. Ensure proper water evaporation when an aliquot of the reaction crude is poked with a capillary, drying the TLC plate well with an air dryer.
When the reaction is complete, cool the flask in an ice bath until the appearance of a white solid, and vacuum filter using a Hirsch funnel. Dry the solid, weigh, determine the melting point, and calculate the yield.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Ethyl acetate | 88.11 | -84 | 77.1 | 0.902 |
| Hexane | 86.18 | -95 | 69 | 0.659 |
| N-Methylmaleimide | 111.1 | 94-96 | - | - |
| 9-(Hydroxymethyl)anthracene | 208.26 | 162-164 | - | - |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Ethyl acetate |   |
| Hexane |     |
| N-Methylmaleimide |   |
| 9-(Hydroxymethyl)anthracene | Non-hazardous |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Ethyl acetate | XEKOWRVHYACXOJ-UHFFFAOYSA-N |
| Hexane | VLKZOEOYAKHREP-UHFFFAOYSA-N |
| N-Methylmaleimide | SEEYREPSKCQBBF-UHFFFAOYSA-N |
| 9-(Hydroxymethyl)anthracene | JCJNNHDZTLRSGN-UHFFFAOYSA-N |
References
Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2