Objective
To produce a cyclic amide (lactam), key in nylon synthesis from a cyclic ketone, via a Beckmann rearrangement.
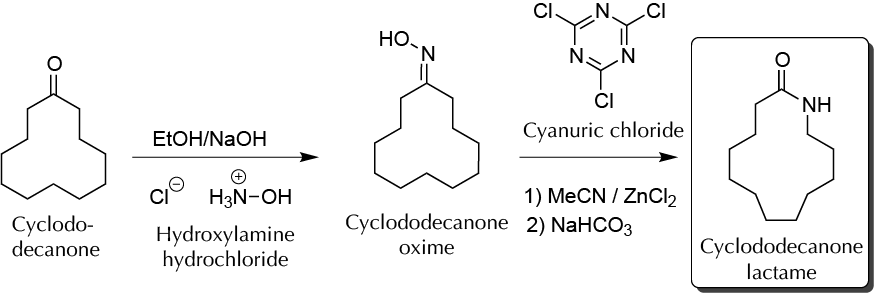
Background
Laurolactam (12-aminododecalactam) is used to synthesize polyamide-12, also known as Nylon-12, which is a semi-crystalline polyamide with very high tenacity and good chemical resistance. In this experiment, laurolactam is synthesized by a process of low environmental impact, in two steps from cyclodecanone, the key step of the synthesis being a Beckmann rearrangement.
Experimental procedure
A) Producing cyclododecanone oxime
In a round-bottom flask, dissolve 1.5 g of cyclododecanone in approximately 8 ml of 9 5 % EtOH. Add 0.6 g of hydroxylamine hydrochloride, 25 ml of deionized water, and 15 ml of aqueous NaOH (10 % by weight), with magnetic stirring. Couple a water condenser and heat the reaction to reflux while stirring. Completion of the reaction is detected by the formation of crystals floating on the surface of the reaction mixture, approximately 30 min after beginning the reflux. After 30 min of refluxing, remove the condenser and cool the mixture in an ice-water bath, until crystallization is complete. Filter the resulting crystals, and dry under vacuum. To purify the product, recrystallize from 95 % EtOH (10 ml EtOH per gram of recovered crystals). Add deionized water (15 ml of water per gram of crystals recovered of crude oxime), and cool the mixture in an ice-water bath until complete crystallization. Filter the solid and, under vacuum, dry the crystals produced in the same way as before.
B) Conversion to cyclododecanone lactam (laurolactam)
To a round-bottom flask, add cyclododecanone oxime produced in the previous step. For this, add 12 ml of a solution of acetonitrile containing 8.0 mg of cyanuric chloride (2,4,6-trichloro-1,3,5-triazine) and 11.5 mg of anhydrous zinc chloride. Using the same reflux equipment as in the previous step, heat the mixture to reflux (82 ºC) for 1 h. Check the completion of the reaction by TLC using as the eluent a mixture of diethyl ether/hexane (1:1). The reaction is complete when the spot of the oxime disappears and a more polar product appears. After the reaction ends, remove the flask from the hot plate, and add 20 ml of an aqueous solution saturated with NaHCO3. Transfer the reaction mixture to a 100 ml separatory funnel, and extract the product with two portions of 15 ml of ethyl acetate. Combine the extracts of ethyl acetate in a 100 ml Erlenmeyer, and dry the ethyl acetate by adding Na2SO4 anhydrous. Filter the drying agent by gravity, collecting it in a tared round-bottom flask, and remove the solvent under reduced pressure (rotary evaporator). Recrystallize from 95 % EtOH (7 ml EtOH per gram of product). Calculate the yield and determine the m.p. (lit. 148–149 ºC). Weigh and calculate the yield.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Acetonitrile | 41.05 | -48 | 81-82 | - |
| Cyclododecanone | 182.3 | 59-61 | 85 | - |
| Cyclododecanone oxime | 197.32 | - | 288.6 | 1.014 |
| Diethyl ether | 74.12 | -116 | 34.6 | 0.71 |
| EtOH | 46.07 | -114.1 | 78.5 | 0.790 |
| Ethyl acetate | 88.11 | -84 | 77.1 | 0.902 |
| Hexane | 86.18 | -95 | 69 | 0.659 |
| Hydroxylamine hydrochloride | 69.49 | 155-157 | - | 1.670 |
| Laurolactam | 197.32 | 150-153 | 348 | 0.973 |
| Na2SO4 | 142.04 | 884 | - | 2.630 |
| NaHCO3 | 84.01 | 300 | - | 2.160 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Acetonitrile |   |
| Cyclododecanone |  |
| Cyclododecanone oxime | Non-hazardous |
| Diethyl ether |   |
| EtOH |  |
| Ethyl acetate |   |
| Hexane |     |
| Hydroxylamine hydrochloride |     |
| Laurolactam | Non-hazardous |
| Na2SO4 | Non-hazardous |
| NaHCO3 | Non-hazardous |
| NaOH |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Acetonitrile | WEVYAHXRMPXWCK-UHFFFAOYSA-N |
| Cyclododecanone | SXVPOSFURRDKBO-UHFFFAOYSA-N |
| Cyclododecanone oxime | SCRFXJBEIINMIC-UHFFFAOYSA-N |
| Diethyl ether | RTZKZFJDLAIYFH-UHFFFAOYSA-N |
| EtOH | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
| Ethyl acetate | XEKOWRVHYACXOJ-UHFFFAOYSA-N |
| Hexane | VLKZOEOYAKHREP-UHFFFAOYSA-N |
| Hydroxylamine hydrochloride | WTDHULULXKLSOZ-UHFFFAOYSA-N |
| Laurolactam | JHWNWJKBPDFINM-UHFFFAOYSA-N |
| Na2SO4 | PMZURENOXWZQFD-UHFFFAOYSA-L |
| NaHCO3 | UIIMBOGNXHQVGW-UHFFFAOYSA-M |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- D. F. Taber and P. J. Straney, The synthesis of laurolactam from cyclododecanone via a Beckmann rearrangement, Journal of Chemical Education 87 (2010), no. 12, 1392–1392, DOI: 10.1021/ed100599q