Written by J.A Dobado | Last Updated on April 22, 2024
Objective
The synthesis of isopropylidene-α-D-glucofuranose, is approached to familiarize the student with some of the most common functional group protection reactions in carbohydrate chemistry. That is, the protection of hydroxyl groups.
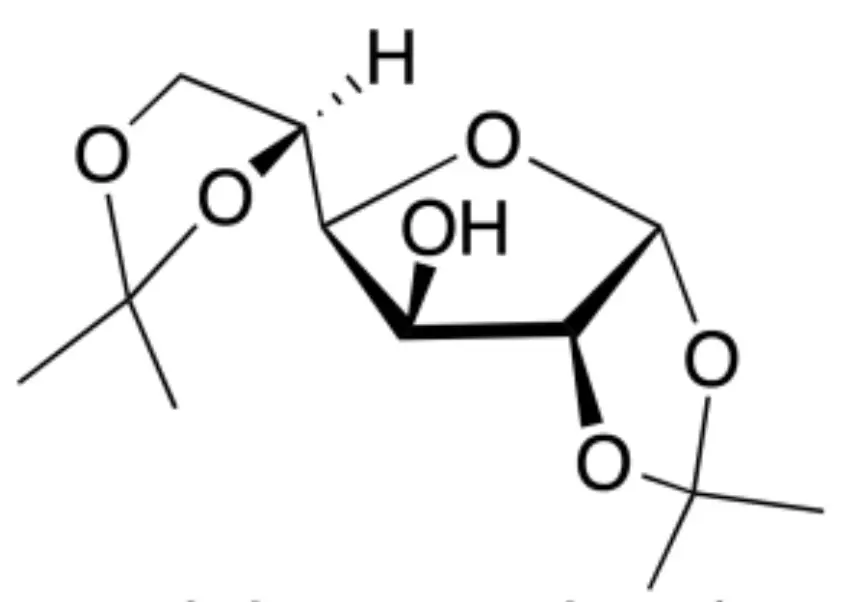 |
| 3D Structure |
Background
Carbohydrates are compounds characterized by having several hydroxyl groups that give characteristic reactions of alcohols. The key step for the synthesis of carbohydrates is the selective functionalization of the different hydroxyls, in order to be able to carry out different reactions on each of them.
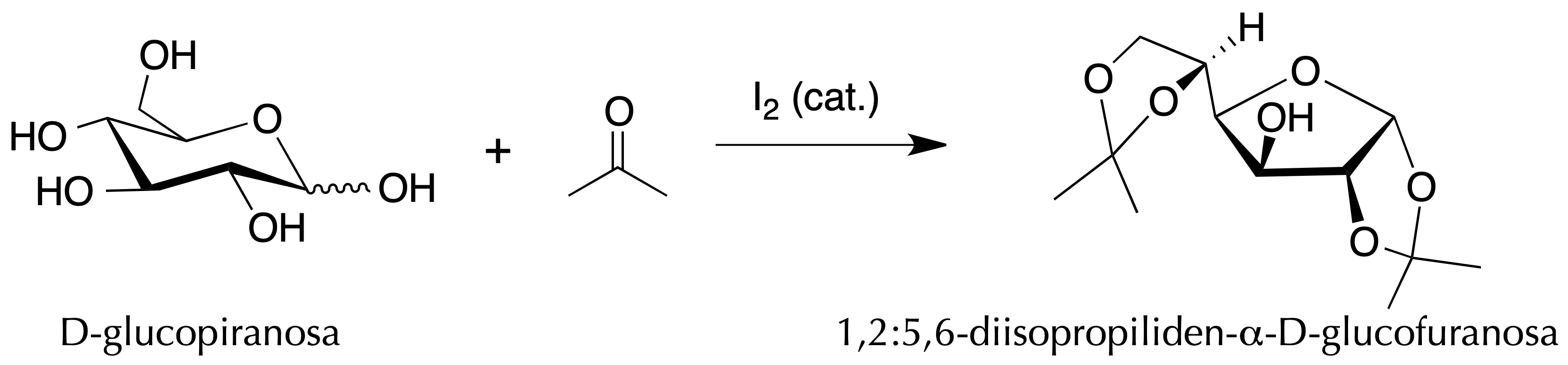
For example, acetone reacts with diols to form acetals. However, in the iodine-catalyzed reaction with D-glucose it is possible to control the conditions so that only the primary and anomeric acetals are formed.
Experimental procedure
To achieve the synthesis of isopropylidene-α-D-glucofuranose, 1.5 g iodine and 5 g glucose are dissolved in 250 ml anhydrous acetone. Then, the mixture, placed in a round bottom flask, is heated at reflux for 2 h. After this time, the mixture is allowed to cool to room temperature.
A saturated aqueous solution of sodium thiosulfate is added to the cold reaction crude, until total decolorization is achieved. The resulting solution is partially concentrated at the rotary evaporator to one third to one quarter of the starting volume.
The solution is allowed to cool and 100 ml of water and 50 ml of chloroform are added. The resulting mixture is transferred to a separating funnel and the organic phase (which is kept) is separated. The aqueous phase is extracted twice with 50 ml of chloroform.
The three organic extracts are combined, dried with anhydrous sodium sulphate, filtered and the filtrate is transferred to a round-bottomed flask and evaporated to dryness at a rotary evaporator. The white solid obtained is the 1,2:5,6-di-O-isopropylidene-α-D-glucofuranose.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| 1,2,5,6-Di-O-isopropylidene-α-D-glucofuranose | 260.28 | 109-113 | - | - |
| Acetone | 58.08 | -94 | 56 | 0.791 |
| Chloroform | 119.38 | -63 | 60.5-61.5 | 1.492 |
| 3-Methyl-butan-1-ol | 88.15 | -117 | 131-132 | - |
| H2SO4 | 98.08 | 3 | - | 1.80-1.84 |
| Iodine I2 | 253.81 | 113 | 184 | 4.930 |
| 1H-Pyrrole | 67.09 | -23 | 131 | 0.967 |
| Na2SO4 | 142.04 | 884 | - | 2.630 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| 1,2,5,6-Di-O-isopropylidene-α-D-glucofuranose | Non-hazardous |
| Acetone |   |
| Chloroform |   |
| 3-Methyl-butan-1-ol |   |
| H2SO4 |  |
| Iodine I2 |   |
| 1H-Pyrrole |    |
| Na2SO4 | Non-hazardous |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| 1,2,5,6-Di-O-isopropylidene-α-D-glucofuranose | KEJGAYKWRDILTF-HOTMZDKISA-N |
| Acetone | CSCPPACGZOOCGX-UHFFFAOYSA-N |
| Chloroform | HEDRZPFGACZZDS-UHFFFAOYSA-N |
| 3-Methyl-butan-1-ol | PHTQWCKDNZKARW-UHFFFAOYSA-N |
| H2SO4 | QAOWNCQODCNURD-UHFFFAOYSA-N |
| Iodine I2 | PNDPGZBMCMUPRI-UHFFFAOYSA-N |
| 1H-Pyrrole | KAESVJOAVNADME-UHFFFAOYSA-N |
| Na2SO4 | PMZURENOXWZQFD-UHFFFAOYSA-L |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- S. Pétursson, Protecting groups in carbohydrate chemistry, Journal of Chemical Education 74 (1997), no. 11, 1297, DOI: 10.1021/ed074p1297