Written by J.A Dobado | Last Updated on April 22, 2024
Objective
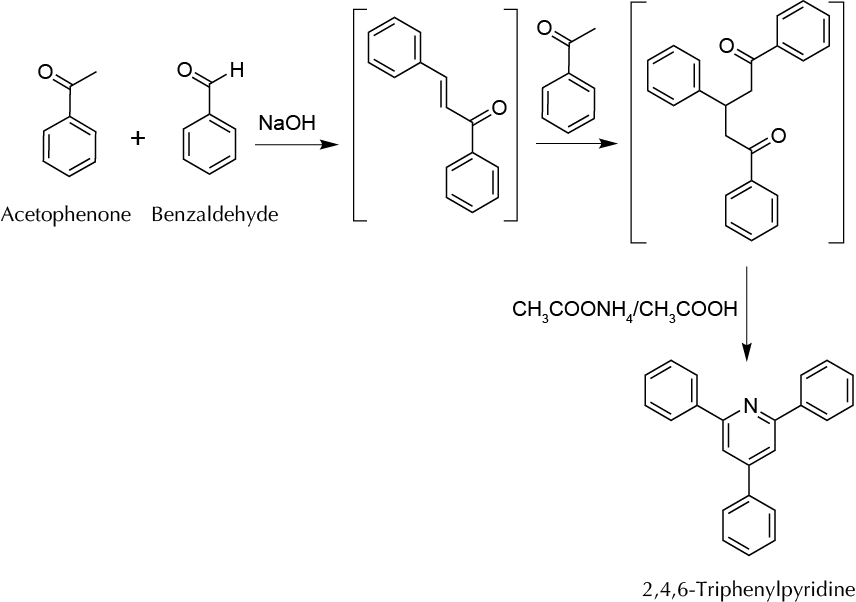
To synthesize a heterocyclic compound (triphenylpyridine) by a sequence of reactions involving an aldolic condensation, a Michael addition, and a final condensation reaction to yield a pyridine ring.
Background
The aldol condensation reaction produces β-hydroxyaldehyde (aldol) or β-hydroxyketones from two carbonylic compounds with at least a hydrogen atom at the α-position with respect to such groups. Starting from two different carbonyl compounds the reaction is called a cross-aldol condensation. In such reactions, usually the primary condensation product is not isolated, since the loss of a water molecule occurs very easily to give α,β-unsaturated carbonylic compounds, active for a Michael addition on the double bond. The experiment described in this practice combines three different reactions that are performed sequentially: aldol reaction, Michael addition, and formation of a pyridine derivative.
Experimental procedure
In a mortar with a pestle, grind and mix an NaOH lentil (they have a mass of between 75 and 95 mg) and 240 mg of acetophenone to form a smooth paste. Then add 110 mg of benzaldehyde. Continue grinding the three reagents for 15 min. Throughout the process, a thick paste forms first and then a solid. Stir and scrape the walls of mortar with a spatula frequently to enhance the mixing process. When finished mixing, let the crude reaction stand for 20 min. Meanwhile, place 150 mg of ammonium acetate and 10 ml of acetic acid into a 25 ml round-bottom flask with a spin vane, stir the mixture at r.t. for 5 min, and then add the solid prepared in the previous step. Fit a water condenser and reflux the mixture for 2 h. Afterward, allow the crude reaction to cool to r.t. To the reaction mixture, add 10 ml of water and cool the flask in an ice-water bath until crystals appear. Filter the resulting solid under vacuum in a Hirsch funnel, and wash it first with deionized water (2 × 3 ml) followed of a solution of 10% sodium bicarbonate (2 × 5 ml). Disconnect the vacuum while adding the sodium bicarbonate, and stir the solid thoroughly with a spatula. Reconnect the vacuum until the liquid disappears completely by suction. Transfer the solid to a Petri dish or watch glass, and when dry, weigh and calculate the yield. The final product can be purified by recrystallization from ethyl acetate (m.p. = 137–138 ºC).
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| 2,4,6-Triphenylpyridine | 307.39 | - | - | - |
| Acetic acid | 60.05 | 16.2 | 118 | 1.049 |
| Acetophenone | 120.15 | 19-20 | 202 | 1.03 |
| Ammonium acetate | 77.08 | 110-112 | - | - |
| Benzaldehyde | 106.12 | -26 | 178-179 | 1.044 |
| Ethyl acetate | 88.11 | -84 | 77.1 | 0.902 |
| NaHCO3 | 84.01 | 300 | - | 2.160 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| 2,4,6-Triphenylpyridine |   |
| Acetic acid |   |
| Acetophenone |  |
| Ammonium acetate | Non-hazardous |
| Benzaldehyde |  |
| Ethyl acetate |   |
| NaHCO3 | Non-hazardous |
| NaOH |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| 2,4,6-Triphenylpyridine | FRZHWQQBYDFNTH-UHFFFAOYSA-N |
| Acetic acid | QTBSBXVTEAMEQO-UHFFFAOYSA-N |
| Acetophenone | KWOLFJPFCHCOCG-UHFFFAOYSA-N |
| Ammonium acetate | USFZMSVCRYTOJT-UHFFFAOYSA-N |
| Benzaldehyde | HUMNYLRZRPPJDN-UHFFFAOYSA-N |
| Ethyl acetate | XEKOWRVHYACXOJ-UHFFFAOYSA-N |
| NaHCO3 | UIIMBOGNXHQVGW-UHFFFAOYSA-M |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |