Written by J.A Dobado | Last Updated on April 22, 2024
Objective
To learn steam-distillation and vacuum-distillation techniques to produce an essential oil from a plant raw material (orange peel).
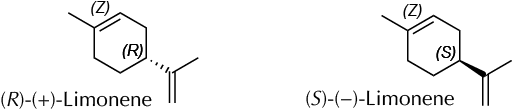
Background
Limonene, a compound of the terpene family, is present in the essential oil of citrus peel. The limonene structure has a chiral center, and thus it is found in nature as two enantiomers the (R)- and (S)-limonene. Isomer (R)- has the characteristic smell of oranges, while the (S)- smells like lemons. In oranges, essential oil comprises 95% of (R)-limonene, whereas lemon peel contains mostly (S)-limonene. Limonene is widely used as a fragrance in cosmetics, a flavoring in the food industry, and even as a biodegradable domestic degreaser in industrial cleaning. Also, it is used as starting material for producing p-cymene by catalyzed dehydrogenation and as an insecticide against ants, aphids, and other pests, (R)-limonene being especially effective.
Experimental procedure
Peel two oranges, striving to have the minimum amount of the white part of the peel. After peeling, chop the skin into small pieces of approximately 1 cm2. Weigh the sample and place the pieces in a 250 ml round-bottom flask with a ground-glass joint, and add 100 ml of deionized water.
Place the flask on a hot plate, insert a stir bar and perform the steam distillation. Among the various configurations of steam distillation, the simplest one (with a Claisen adapter, addition funnel, distillation head, thermometer, condenser, extension, and collector) should be chosen.
Connect the hot plate, regulating the temperature so that the dropping rate of the distillate is approximately 20 drops/min. Add water from the addition funnel so that the volume of liquid in the flask is kept constant, to prevent the sugars contained in the orange peel from caramelizing in the heat. Collect the distillate (approximately 100 ml), which will appear as an emulsion.
After the distillation, the content of the collector is transferred to a separatory funnel. Add CH2Cl2 (2 × 25 ml) and decant. Combine the organic phases and dry with anhydrous Na2SO4. Remove the desiccant by gravity filtration. Pour the CH2Cl2 onto a round-bottom flask, previously dried and tared, and remove the solvent under reduced pressure in a rotary evaporator. Weigh the flask and determine the percentage of essential oil of orange peel.
Limonene thus distilled can be purified by vacuum distillation. Temperature must be carefully controlled with efficient magnetic stirring to prevent splashes. The distilling flask must be cooled with an ice bath. Apply vacuum with a water pump (approximately 20 mm Hg). The distillation temperature for limonene is 75–80 ºC (at a pressure of 3 to 5 Torr, is 35–38 ºC).
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| CH2Cl2 | 84.93 | -97 | 40.0 | 1.33 |
| Na2SO4 | 142.04 | 884 | - | 2.630 |
| R-(+)-Limonene | 136.23 | - | 176-177 | - |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| CH2Cl2 |  |
| Na2SO4 | Non-hazardous |
| R-(+)-Limonene |    |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| CH2Cl2 | YMWUJEATGCHHMB-UHFFFAOYSA-N |
| Na2SO4 | PMZURENOXWZQFD-UHFFFAOYSA-L |
| R-(+)-Limonene | XMGQYMWWDOXHJM-JTQLQIEISA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- J. H. Beatty, Limonene —a natural insecticide, Journal of Chemical Education 63 (1986), no. 9, 768, DOI: 10.1021/ed063p768