Written by J.A Dobado | Last Updated on April 22, 2024
What are alkenes?
Alkenes are compounds that have at least one C=C double bond. They are also known as olefins.
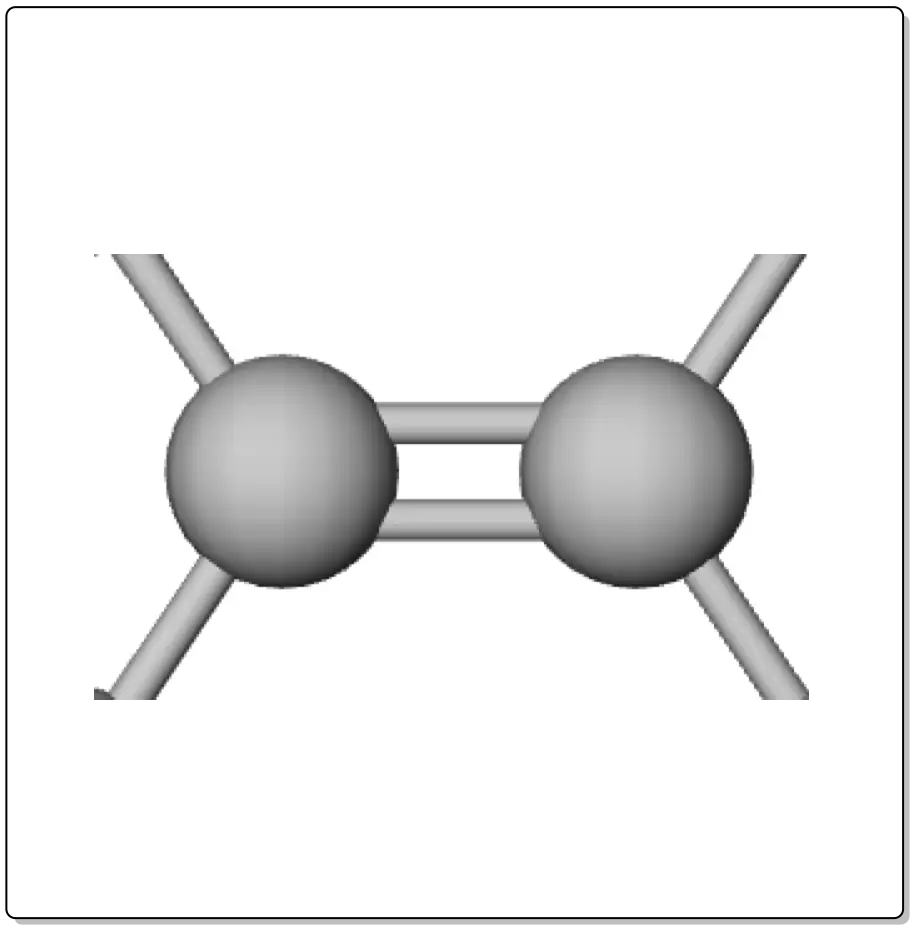
When there is only one double bond in the molecule, its general formula is CnH2n, and for each additional double bond, two hydrogens are subtracted from the formula.
The carbons connected by a double bond have sp2 hybridization, so the double bond is made up of a σ bond (formed by the overlap of two sp2 orbitals) and a π bond (formed by the overlap or delocalization of 2 electrons between the unhybridized pz orbitals.
Nomenclature
Follow the link for a summary of the formulation and nomenclature rules for alkenes.
Reactions
The different nature of these >C=C< double bonds within the molecule is responsible for the special reactivity of alkenes. These reactions are detailed in the section on alkene reactions.
Analysis of alkenes
The most characteristic properties of alkenes are based on the presence of double bonds (unsaturations).
The main features include:
- The IR spectrum is considerably more complex than in saturated hydrocarbons. The >C=C< absorption appears in the region 1680-1620 cm-1.
- Charge transfer test with iodine: The addition of several drops of test liquid to a small iodine crystal will give a brown solution for the positive test.
- They are at least partially soluble in concentrated H2SO4.
Bromine assay in CCl4
Procedure: Dissolve 2 drops (or about 50 mg) of product in 1 ml of CCl4 and add dropwise a 2 % bromine solution in CCl4 until the bromine color persists. If it is necessary to add more than two drops for the color to persist, the assay is considered positive.
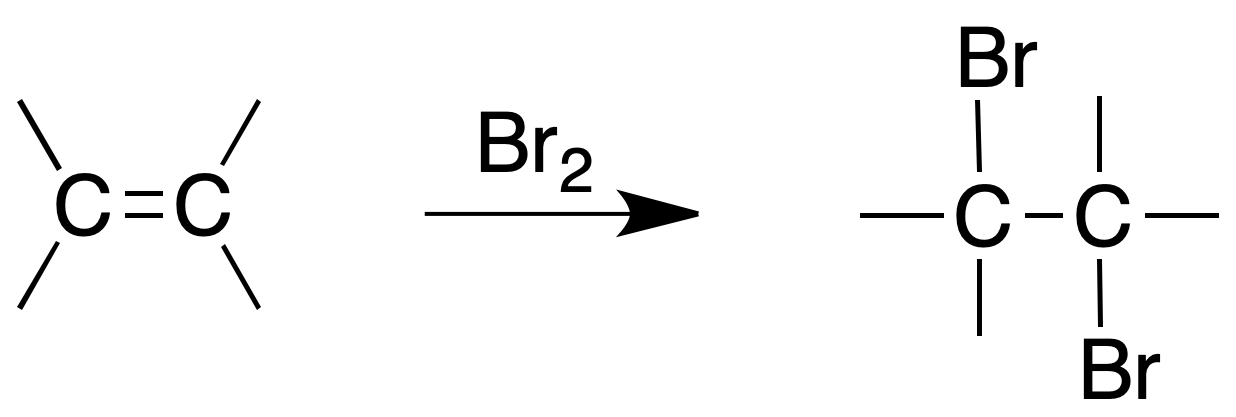
Alkynes also give this reaction. Therefore, the same test must also be checked with a substance that does not add bromine.
In the case of a substitution reaction, gaseous hydrobromic acid (HBr) would be released.
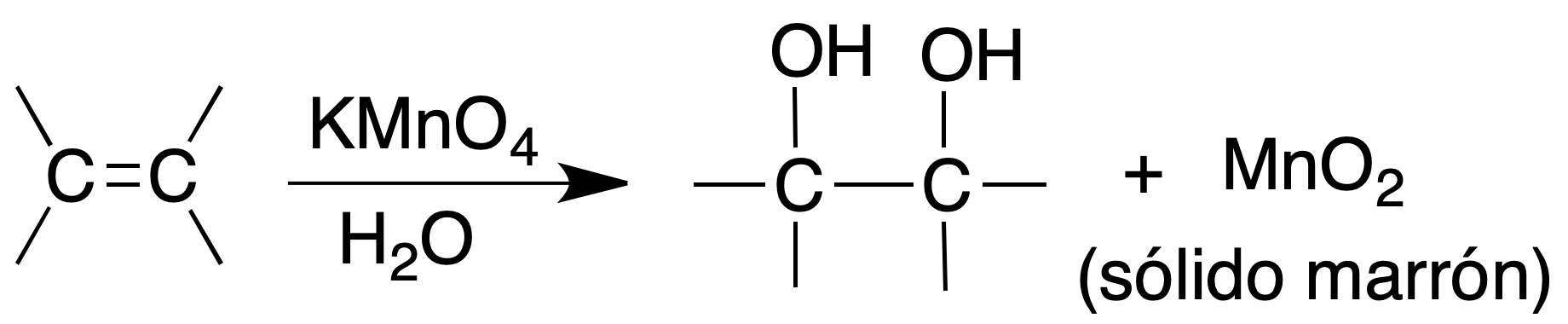
Baeyer’s test (potassium permanganate test)
Dissolve a drop (or 25 mg) of the compound in 2 ml of water or acetone in a test tube. To add drop by drop and shaking strongly an aqueous solution of potassium permanganate to 1 %. The result is positive if it is necessary to add several drops so that the violet color persists (of the KMnO4 on the brown color of the precipitate).
Articles on the website