Written by J.A Dobado | Last Updated on April 22, 2024
What are indoles?
The indole unit occurs in nature in a great variety of structures. Thus, more than 100 alkaloids derived from indole are known. In addition, many of these natural compounds have a relevant physiological activity, for example, in the treatment of leukemia and cancer.
Indole is a volatile crystalline solid with a melting point of 52 ºC and a persistent odor. Its properties are those of an aromatic compound. Much of the chemistry of indole is analogous to that of pyrrole.
For example, it is weakly basic and the stable cation is formed by protonation of carbon. Electrophiles attack the 5-membered ring rather than the 6-membered ring.
Several methods for indole synthesis have been described and are summarized below:
Fischer indole synthesis
Bischler indole synthesis
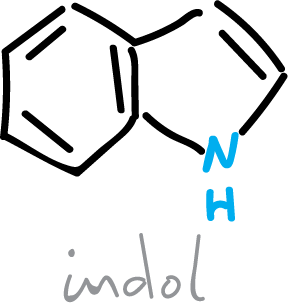
The Bischler synthesis of indole is effected by the reaction of an arylamine with an α-halo-, α-hydroxy-, or α-arylamino-ketone compound, in the presence of acid.It has particular utility for preparing identically substituted indolesin positions 2 and 3.
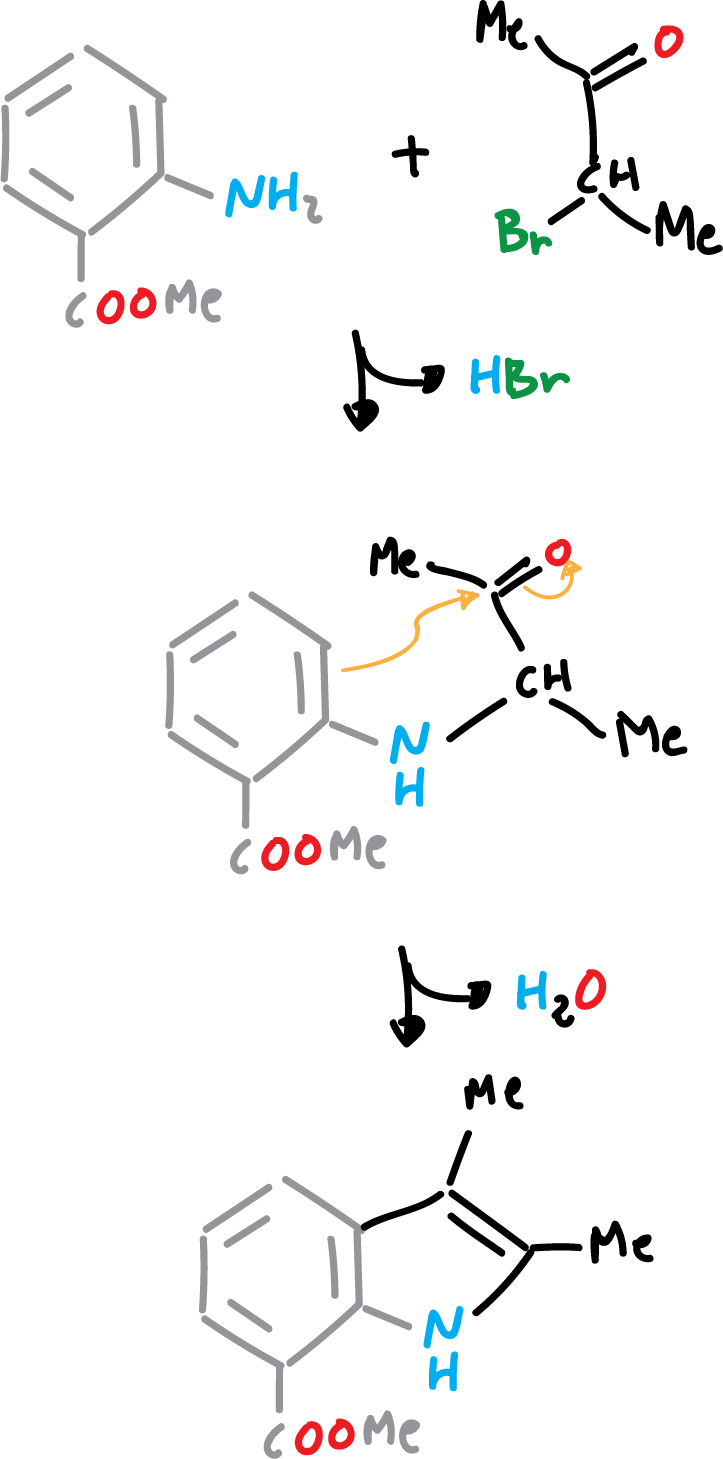
Indole Reissert Synthesis
The Reissert synthesis of indole consists of acylation with diethyl oxalate of the methyl group, activated from o-nitro-toluene, followed by reduction and cyclization.
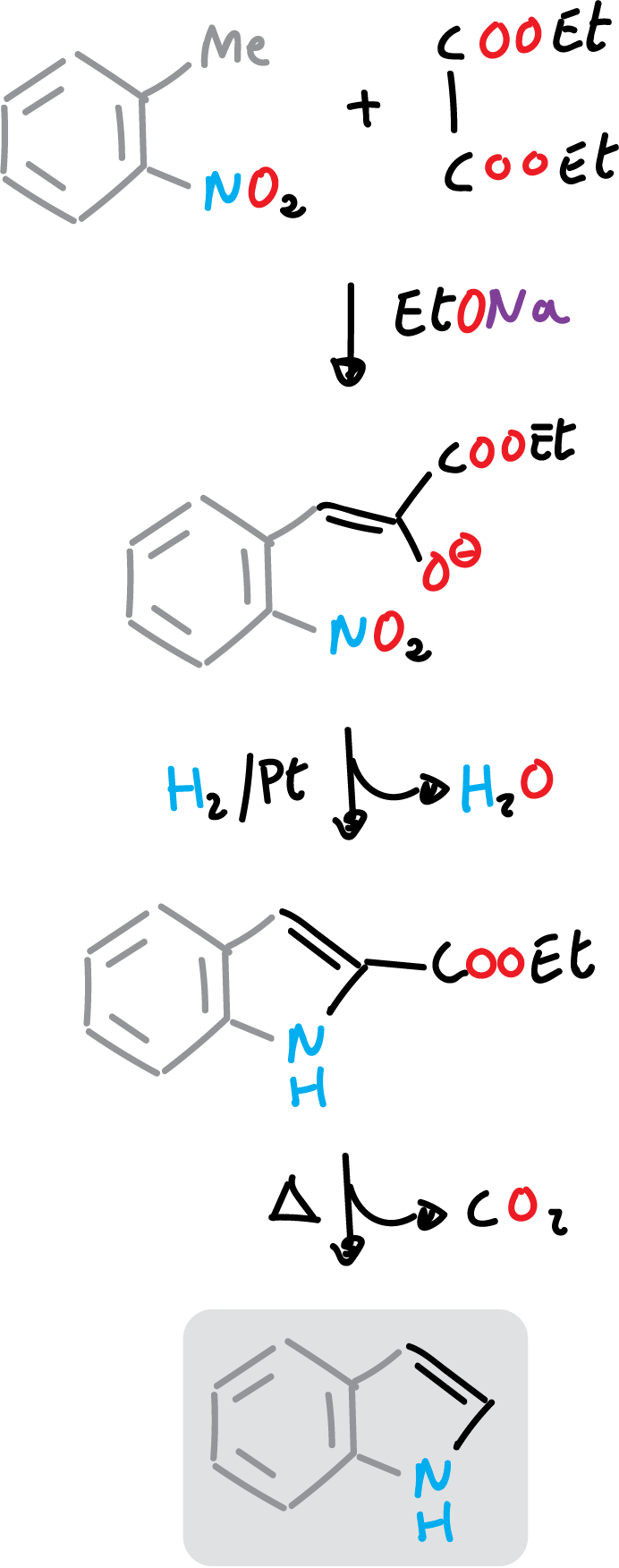
In addition, the ester group remaining on the indole derivative can be hydrolyzed and decarboxylated, as shown in the figure.
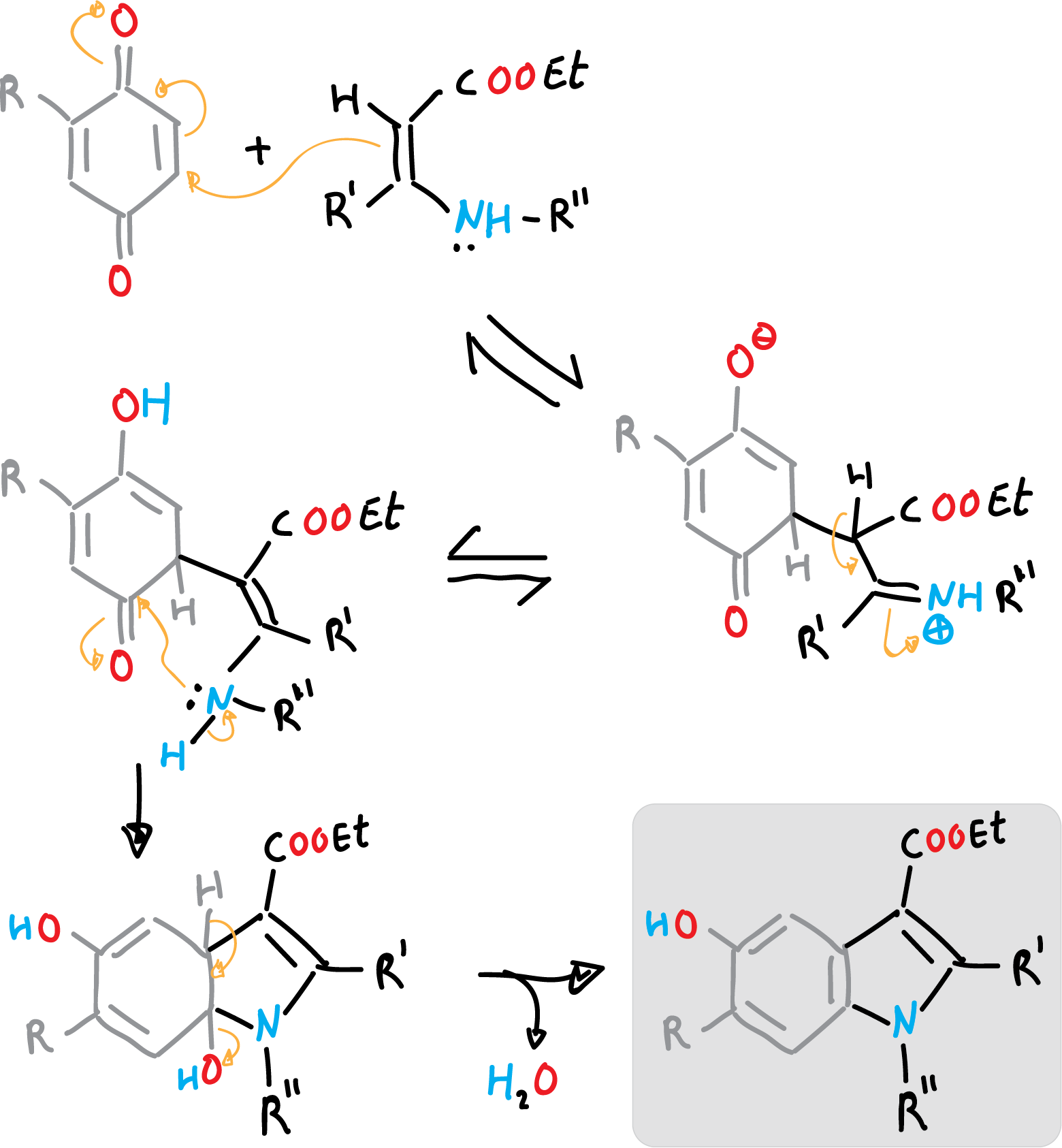
Nenitzescu synthesis of indole
Nenizescu’s synthesis of indole is carried out by consensation of 2-methyl-1,4-benzoquinone with ethyl 3-methylamino-chrotonate. Furthermore, the 5-hydroxy-indole obtained are biologically active compounds.
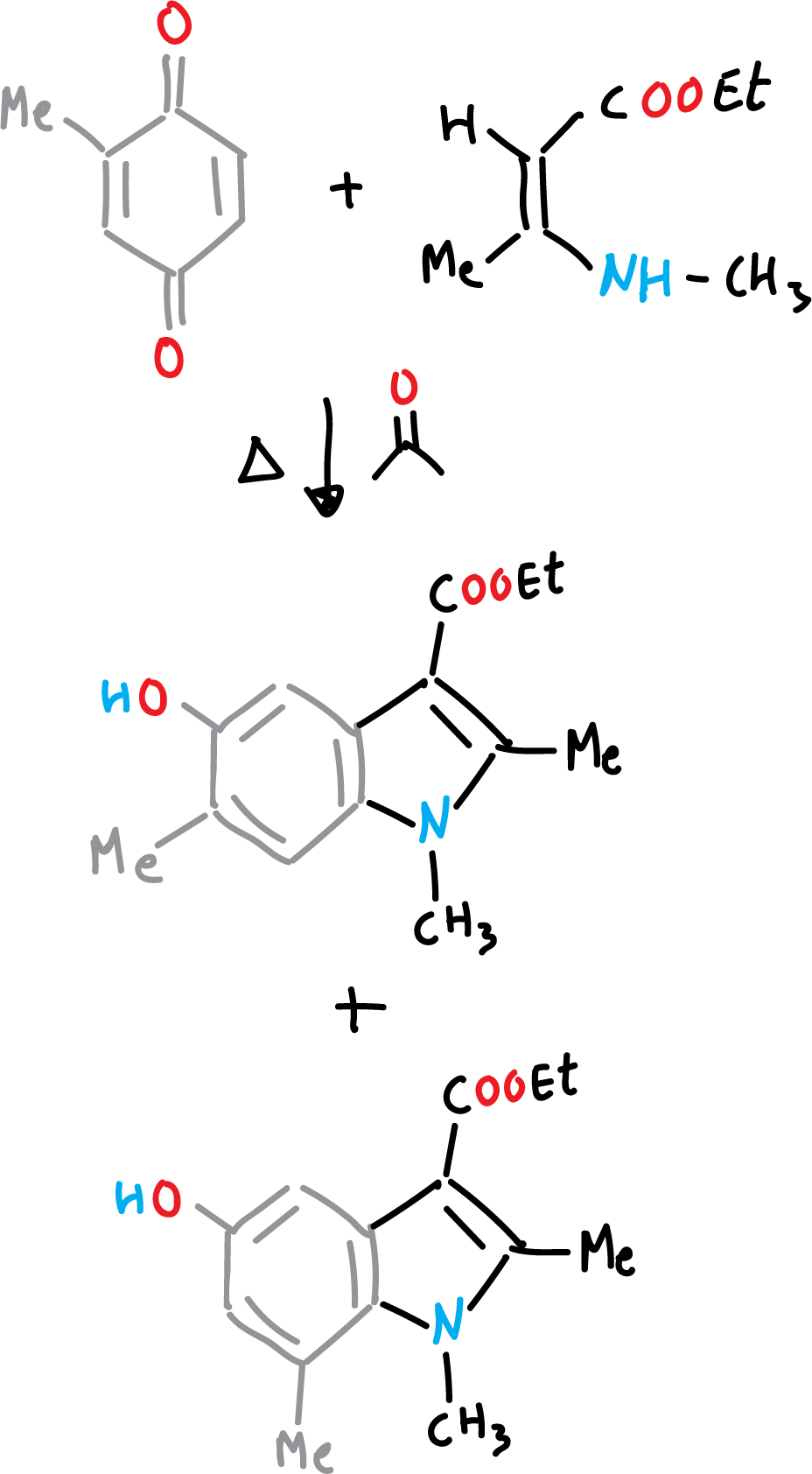
The reaction mechanism can be described as a Michael-type addition, from enamine to benzoquinone, followed by cyclization, and a final dehydration step.
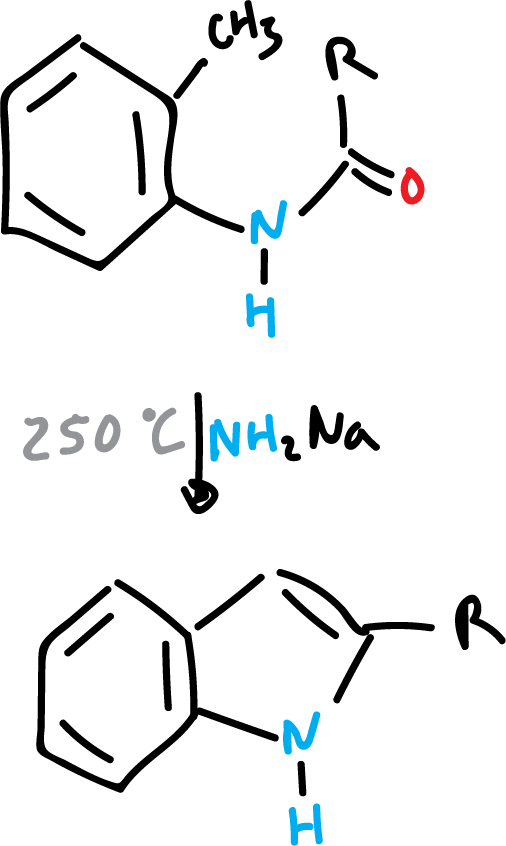
Madelung indole synthesis
The Madelund synthesis proceeds by cyclization dehydration of acyl o-toluidines, N-acyl o-toluidines, or o-aminotoluenes with strong bases at high temperatures to produce indoles. This reaction is carried out in the absence of air.
There is another variant which consists of performing it at room temperature with n-butyllithium as a base. In this way, other R groups can be introduced into the structure to form more complex indoles.
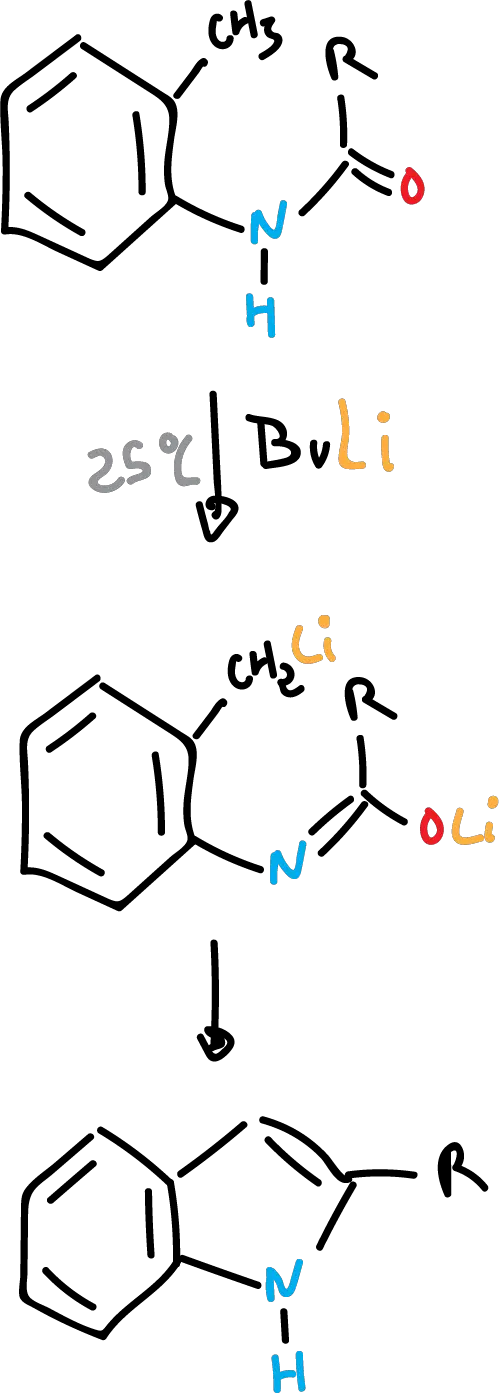
Reactivity
Metallated indoles (acidity)
Indole is a weak acid (with a value of pKa = 16.97), with an acidity comparable to pyrrole, and to aliphatic alcohols. It can be converted to the N-sodium derivative, in liquid ammonia, or with sodium hydride (NaH) in an organic solvent.
On the other hand, indole can give indolyl salts by abstraction of the proton from the nitrogen by means of strong bases or Grignard reagents.
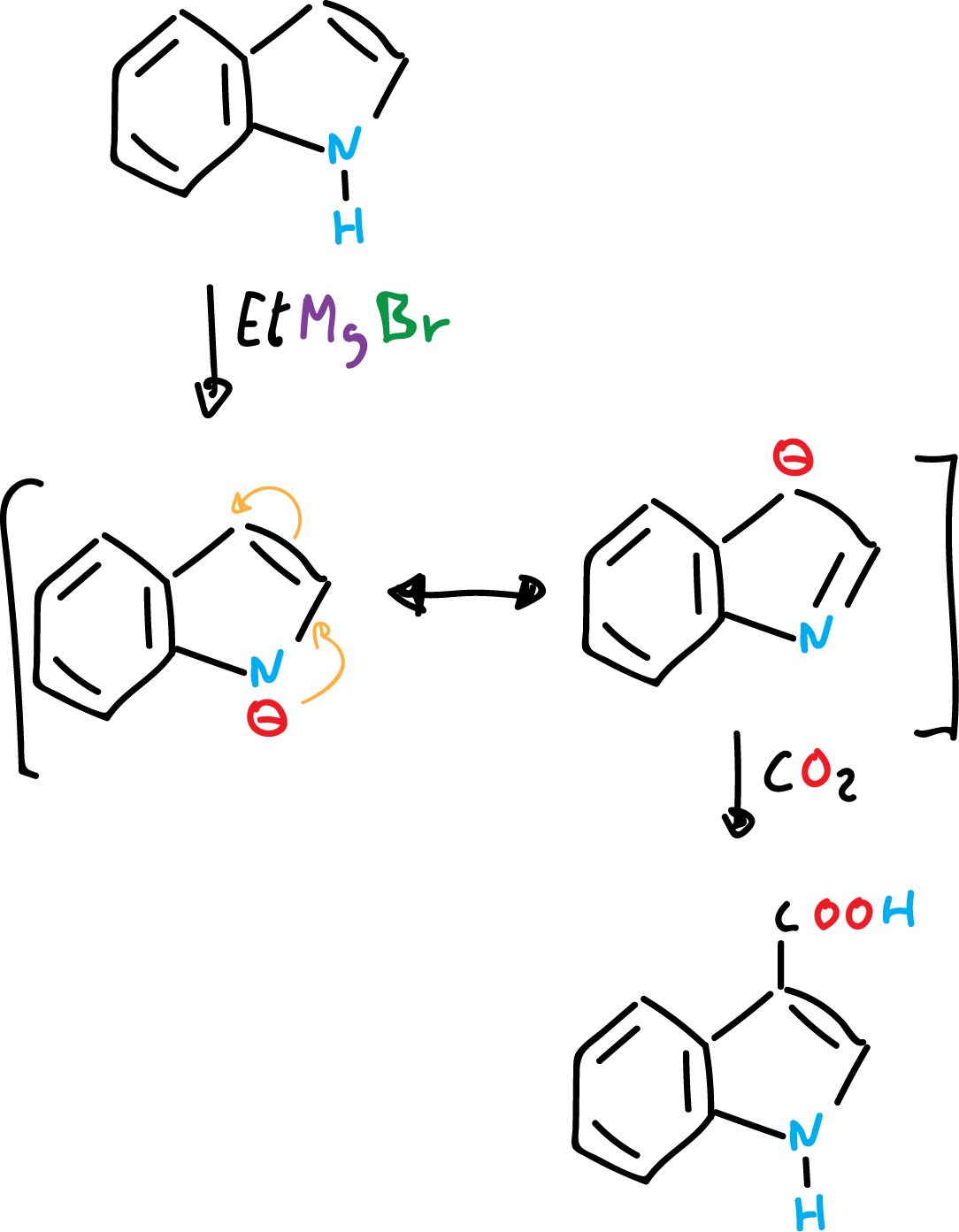
The indolyl salts can be alkylated at nitrogen or at carbon C3, depending on the temperature, the solvent and the nature of the cation (positive ion) involved. In general, alkylation is favored at C3, especially at high temperatures.
Electrophilic substitution of indoles
Indole is a nucleophilic heterocycle and reacts very easily with electrophiles. In this case, unlike pyrrole, the preferred position is on C3.
This can be explained by the fact that a nucleophilic attack at the C2 position leads only to a carbocationic intermediate (preserving the aromaticity of the benzene ring). However, for the attack in C3 position, two resonant structures can be written (keeping aromaticity of the benzene ring), being therefore more stable an attack in C3.
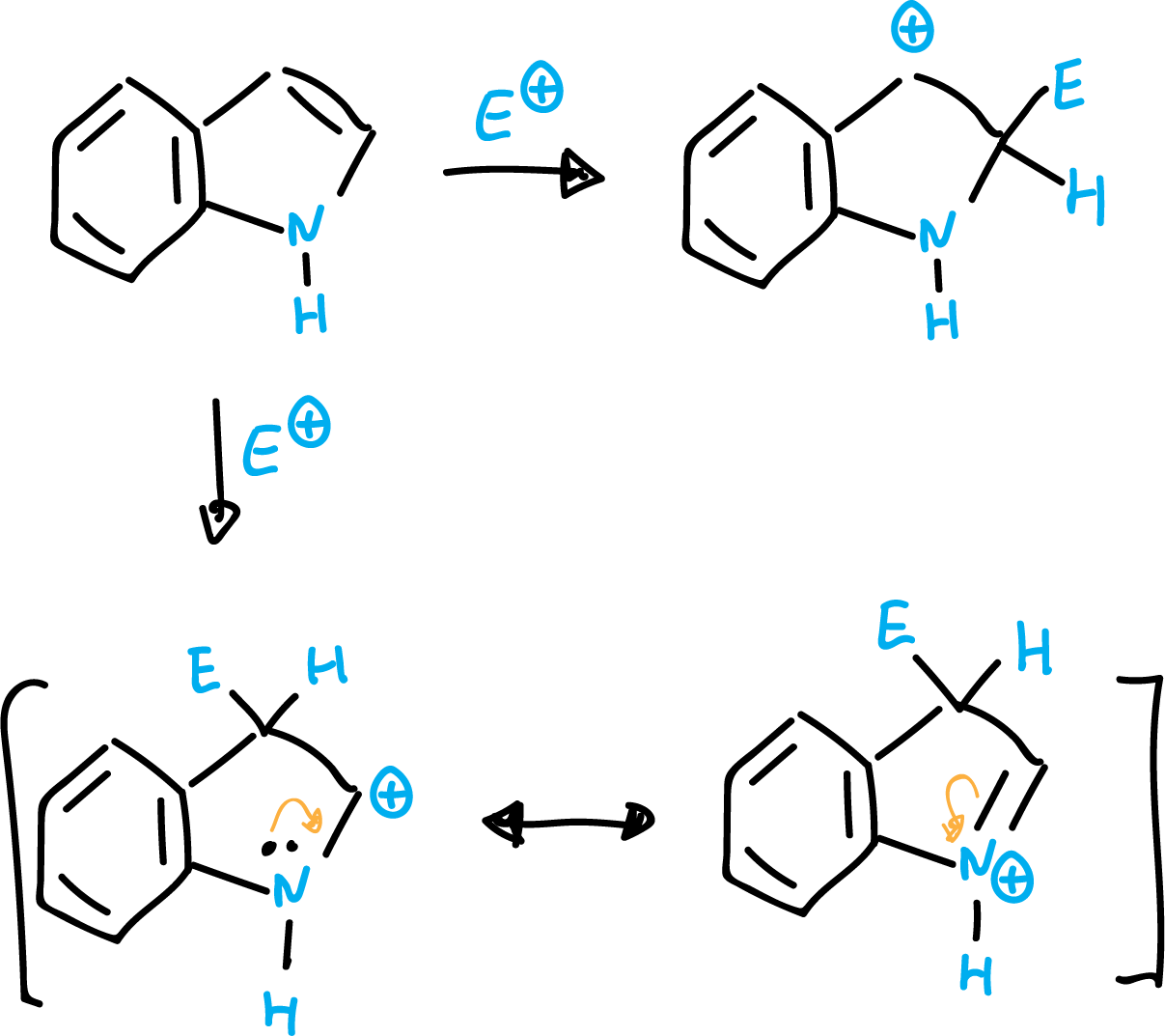
Indole is a very weak base and the most stable cation results from protonation at the C3 carbon rather than at the nitrogen.
Indoles as alkyl substituents at C3 are also preferably protonated at the 3-alkylated position.
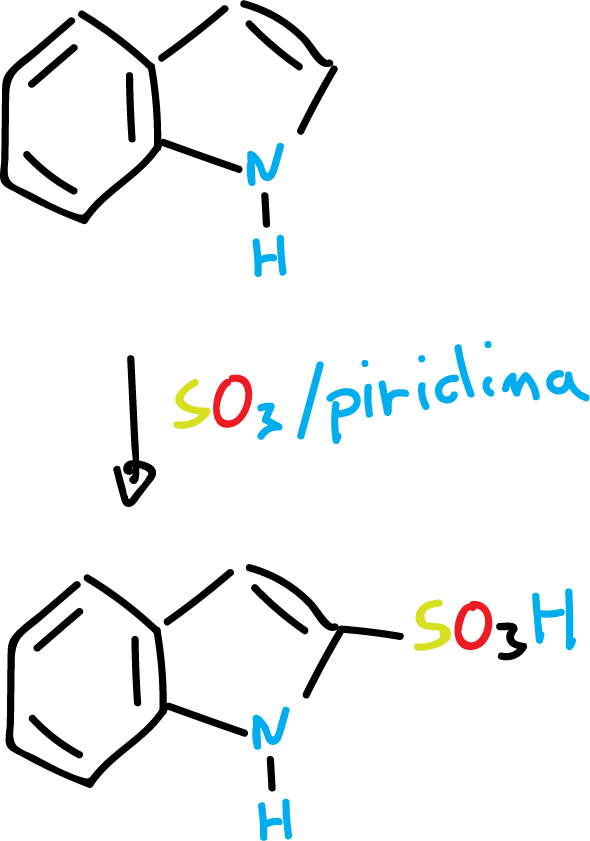
For example, the sulfonation reaction is carried out under mild conditions, using SO3 and pyridine solvent, at C3, as indicated in the scheme.
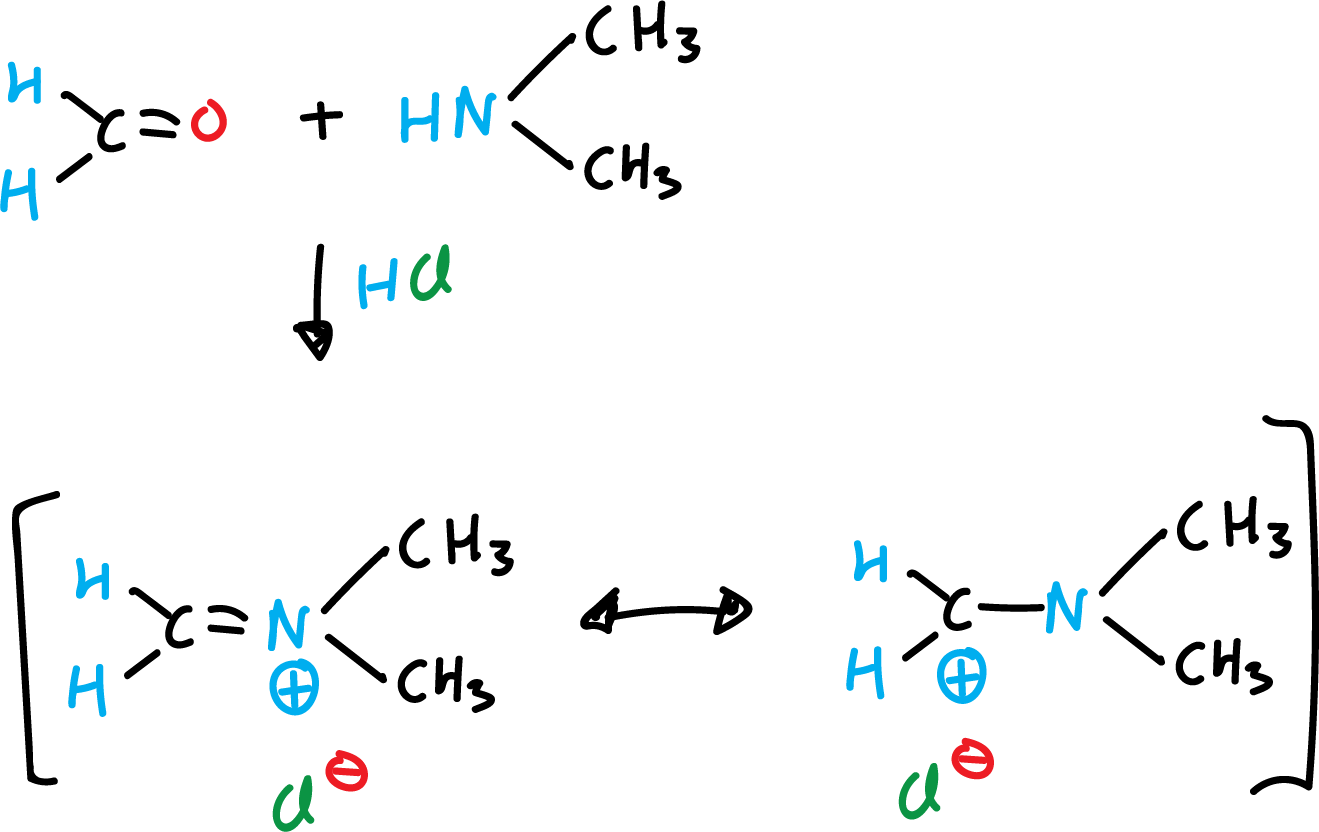
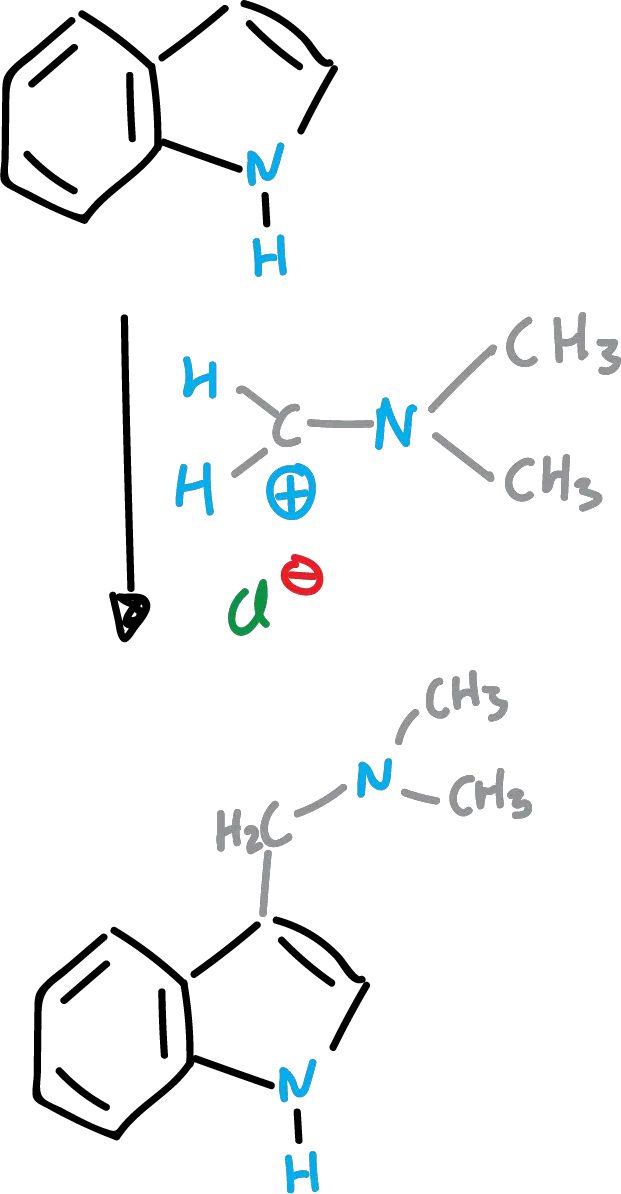
Mannich reaction of indoles
Michael reaction of indoles (or Michael addition)
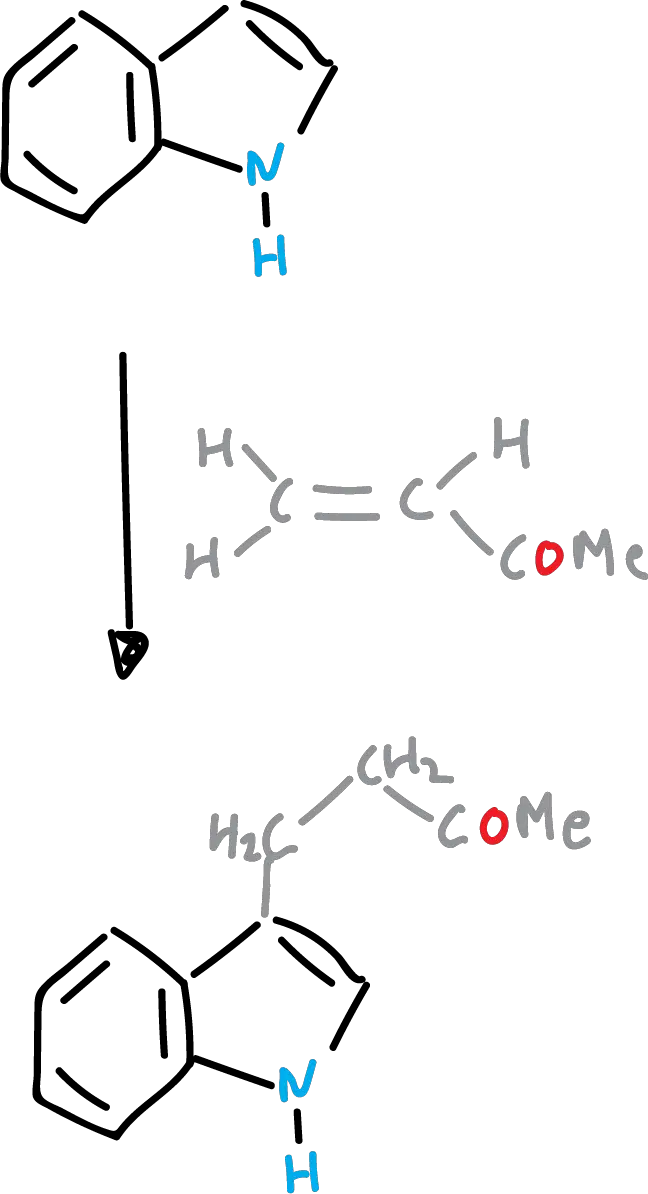
Friedel-Crafts reaction of indoles (or Friedel-Crafts acyclization)
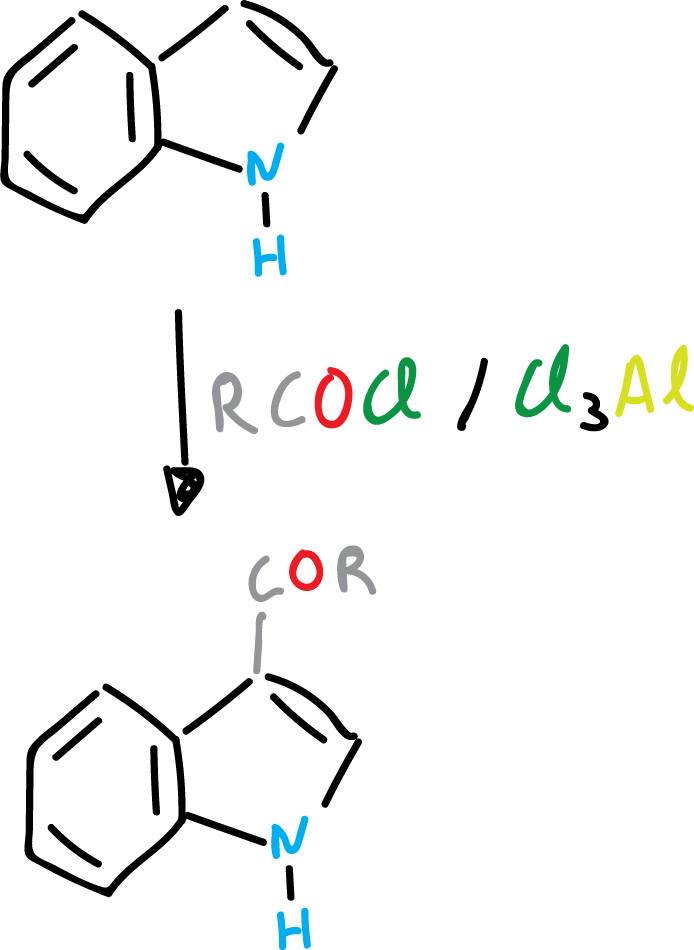
In electrophilic substitution, it may happen that the C3 position of the indole is occupied. Then, it is still the heterocycle that is substituted and it does so at the C2 position.
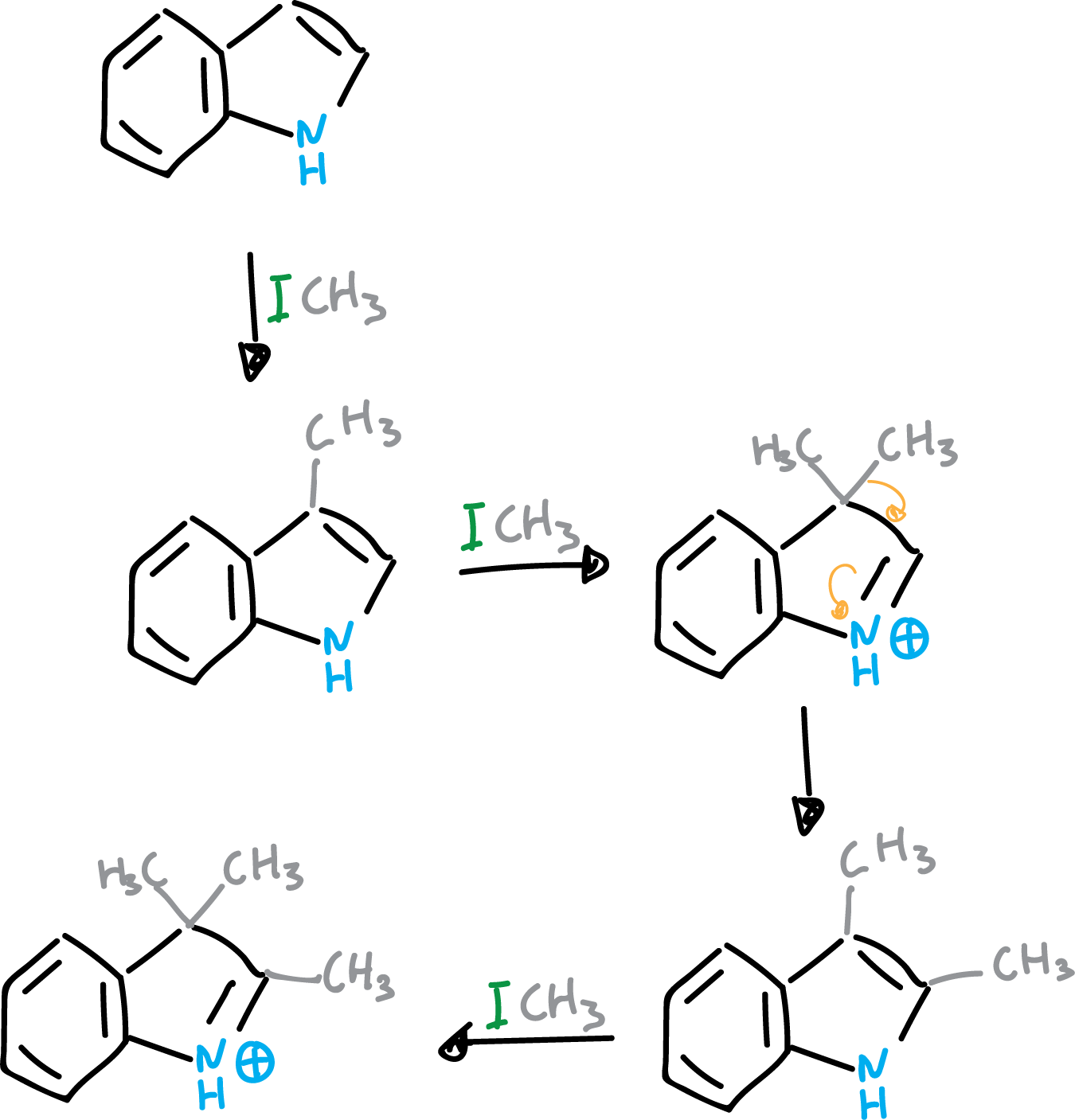
The mechanism involves a substitution at C3, giving rise to a 3,3-disubstituted compound which subsequently migrates. If a thorough methylation of the indole is carried out, the product obtained is as shown in the figure.
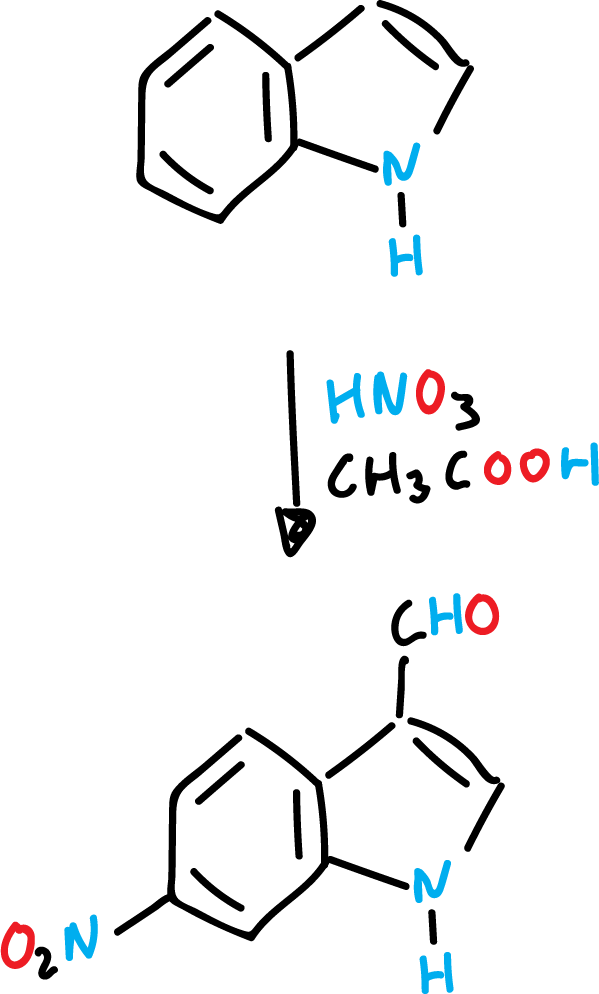
When the indole has an electrophilic substituent on the heterocyclic ring (such as –CHO), the heterocyclic ring is deactivated. Then, the attack on the benzene ring occurs.
On the other hand, when the two indole positions (C2 and C3) are blocked, a substitution usually occurs in the benzene ring, as shown in the scheme.
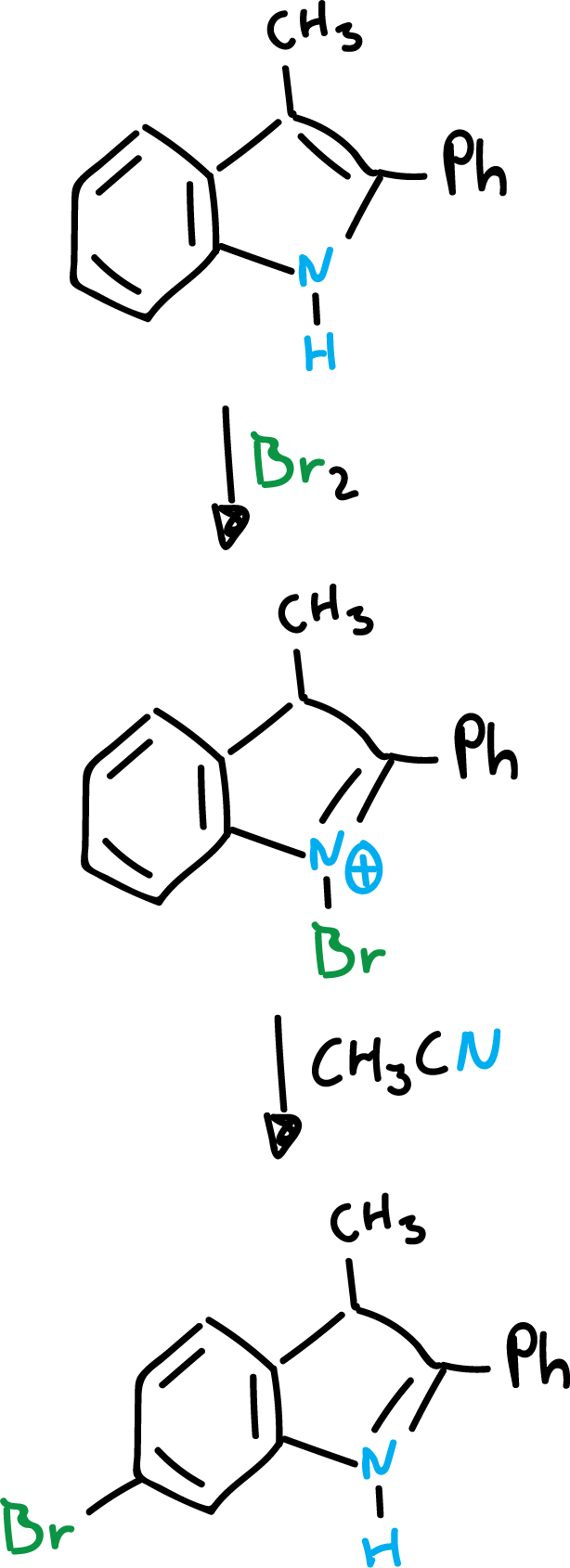
Finally, the presence of an amino or hydroxyl function in the benzene ring, will orient exclusively to the benzene ring, even if the other positions are empty.

Indole nitration
Indole nitration is very sensitive to the reaction medium. Thus, with concentrated nitric acid (HNO3) and glacial acetic acid (CH3COOH) the expected product is formed in position C3.
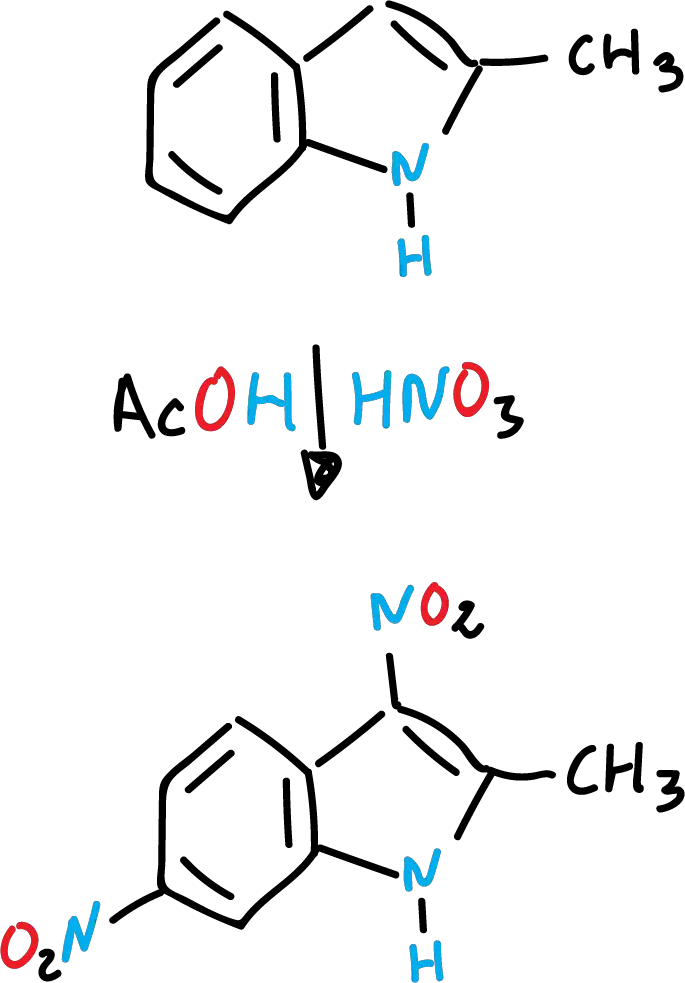
However, in sulfuric acid (H2SO4), nitrated derivatives are produced almost exclusively at the C5 position (on the benzene ring).
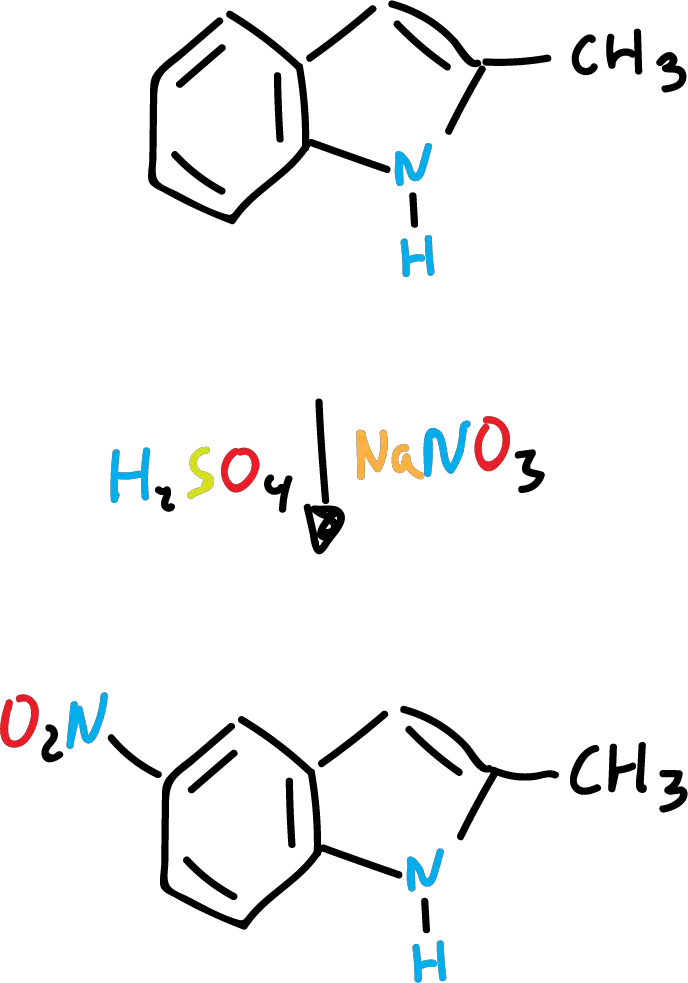
A possible explanation could be that in the case of nitration it is carried out on the free indole, whereas with sulfuric acid, it is carried out by means of an intermediate (conjugated acid).
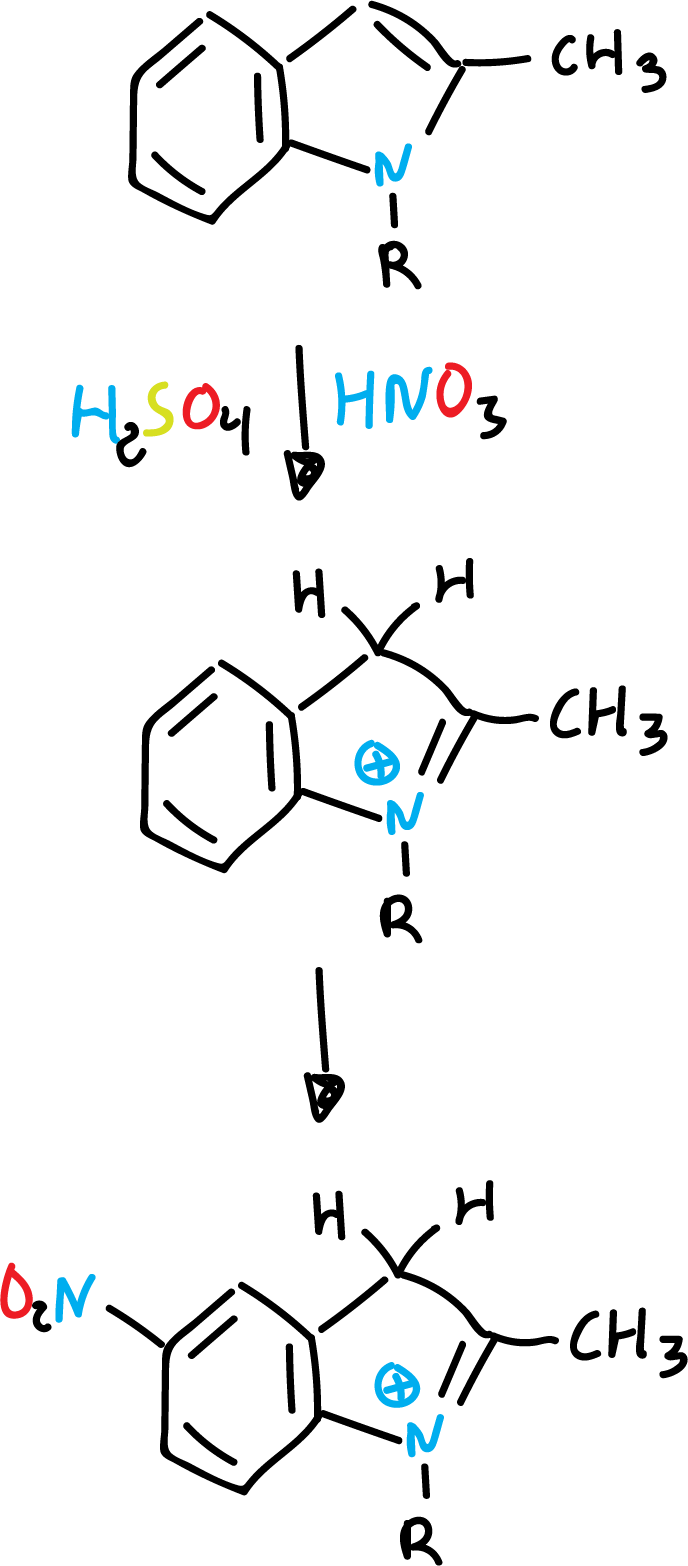
Thus, the protonated heterocycle is deactivated against (E⊕) electrophiles.
Autooxidation reactions of indoles in a basic medium
Electrophilic substitution or addition
The reaction is carried out in the presence of oxygen and a strong base such as potassium terbutoxide (ButO⊖K⊕).
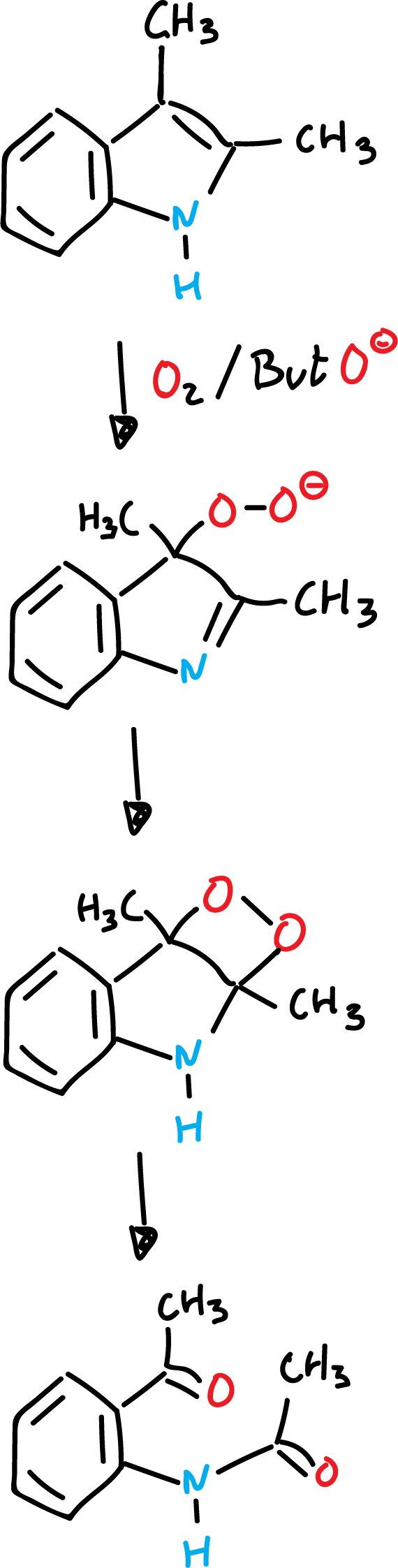
When the dioxetanic ring is opened in the presence of suitable reagents, chemiluminescence originates.
Addition reactions
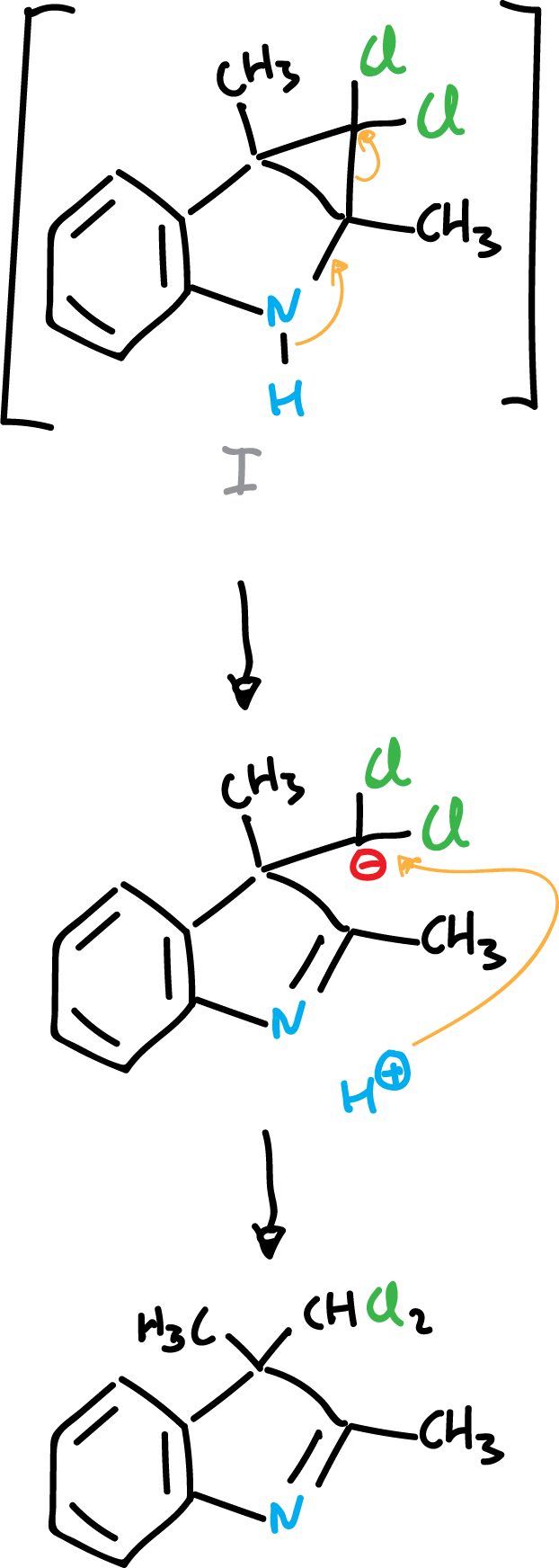
When indole derivatives are reacted with electron-deficient species, such as carbenes, addition onto the C2=C3 double bond can occur.
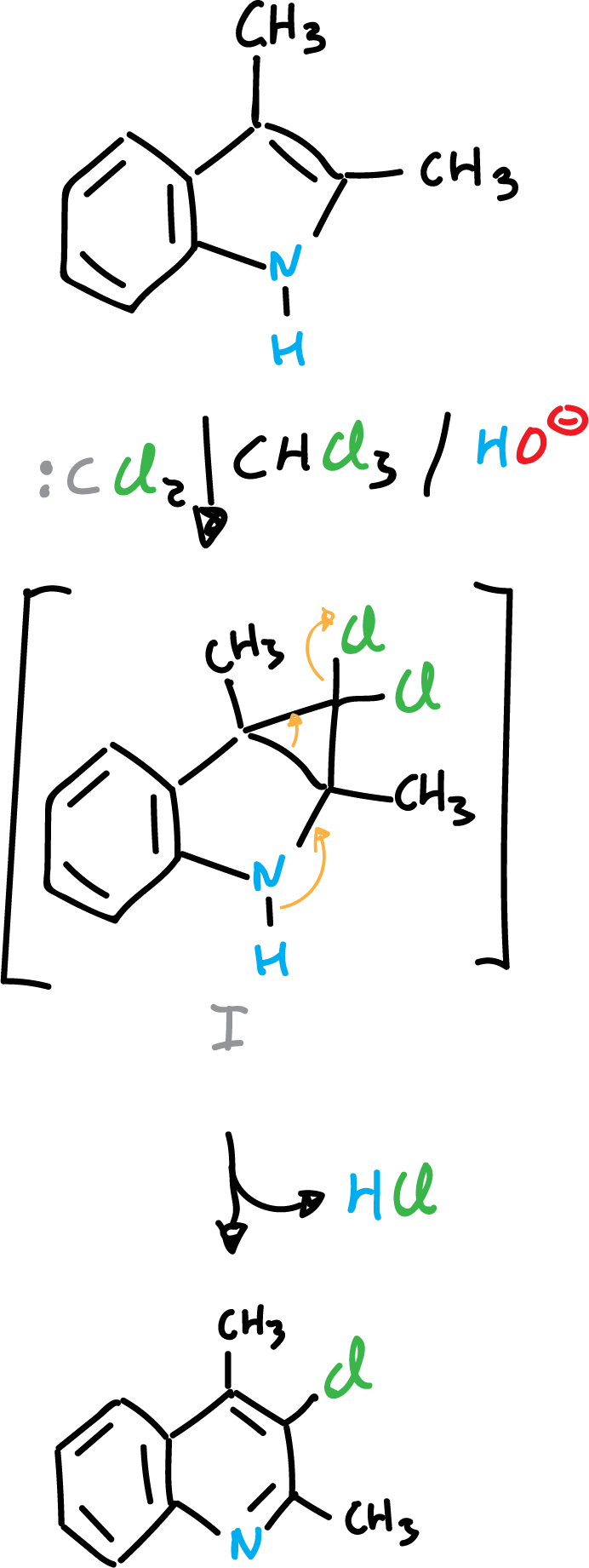
or the reaction proceeds to the next product:
Properties of some substituted indoles
The hydroxy derivatives at C2 and C3 of indoles exist mainly in the keto form.
If the hydroxy derivative is in the C2 position it gives rise to amides.
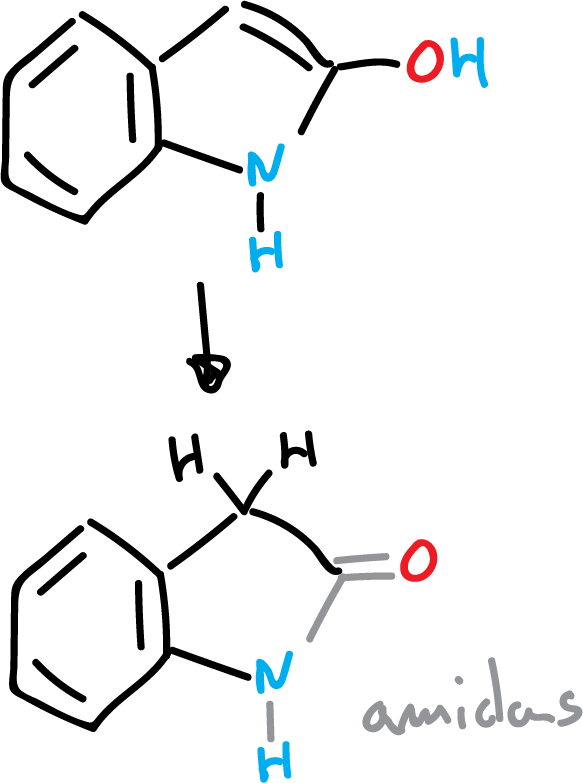
However, if the hydroxy derivative is present in the C3 position, the carbonyl compound called indoxyl is obtained.
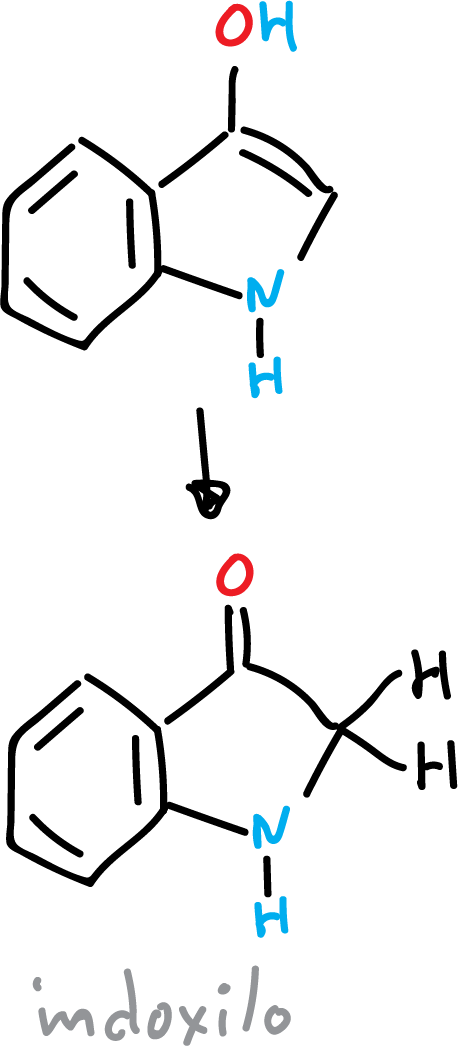
The protons of the carbon atoms adjacent to the carbonyl group are very acidic.
Indoxyl is very unstable due to its tendency to form a radical by removal of an (H·).
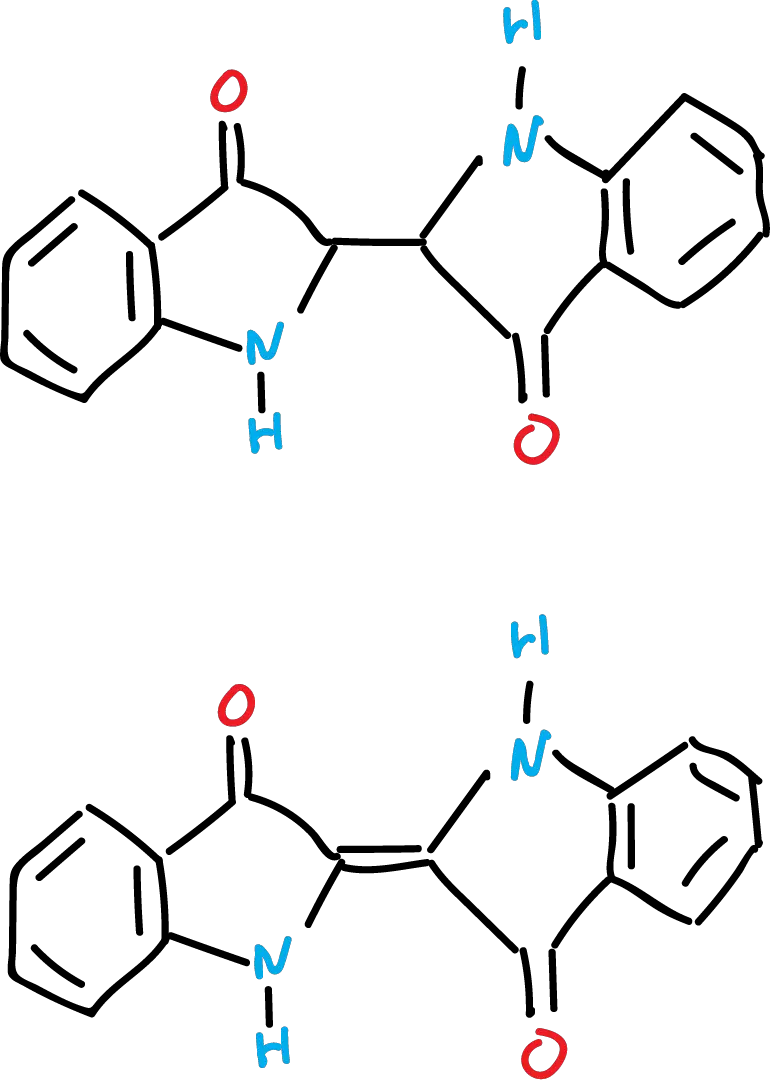
These radicals are relatively stable and couple to give the dimer, which by subsequent oxidation we obtain the indigo colorant (blue in color).
Reactivity of 2-methyl indoles
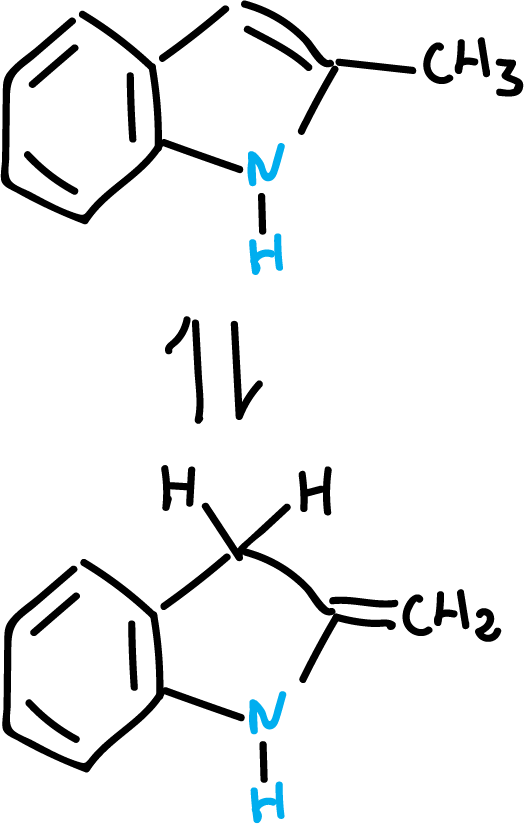
Methyl protons are more acidic.
In addition, the methyl group may be forming the following tautomeric equilibrium.
On the other hand, indole carboxylic acids in C2 and C3 positions decarboxylate when heated in acid solution. Moreover, in this case the protonation at C3 favors this reaction.