Written by J.A Dobado | Last Updated on April 22, 2024
In the analysis of sulfur compounds (thiols), due to the different oxidation states of sulfur, there are many compounds that contain sulfur: thiols, sulfides, disulfides, sulfoxides, sulfones, sulfenic, sulfinic and sulfonic acids, etc.
Their exhaustive study is beyond the limits of this page. Only as an example we will deal with thiols.
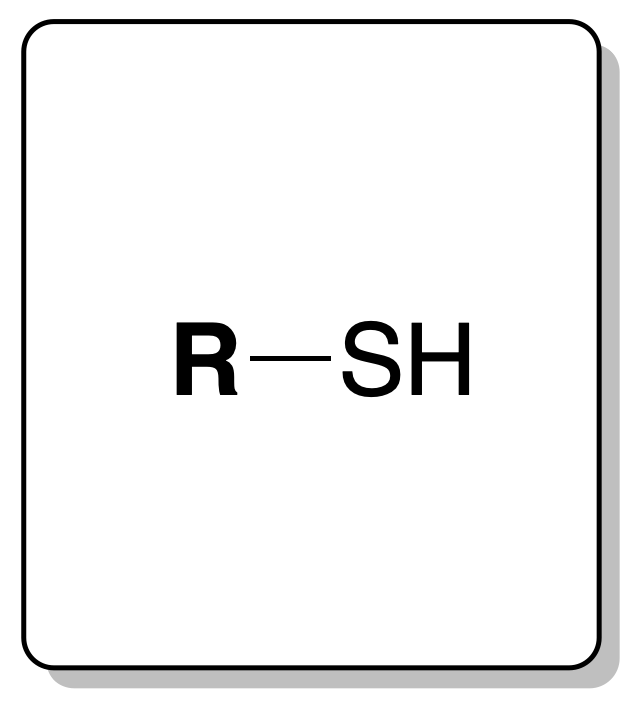
They have an odor reminiscent of hydrogen sulfide (SH2). They are usually insoluble in water but soluble in aqueous alkalis forming soluble salts. They produce a yellow precipitate when a few drops of the thiol are added to a solution of lead acetate in EtOH.