Written by J.A Dobado | Last Updated on April 22, 2024
Objective
The purpose of this experiment is to obtain an alcohol and carboxylic acid in one step using the Cannizzaro reaction on benzaldehyde (autooxidation-reduction reaction or dismutation of an aromatic aldehyde).
Background
Addition of dilute bases to aldehydes or ketones leads to the formation of β-hydroxy aldehydes or β-hydroxyketones by aldol condensation. When the aldehyde or ketone has no α-hydrogens, aldol condensation does not take place, but undergoes autooxidation-reduction in the presence of strong bases giving an equimolecular mixture of the corresponding alcohol and acid (the latter in salt form).
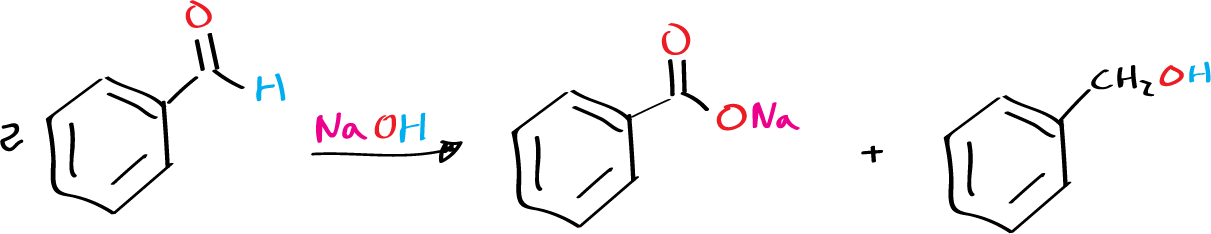
For example, benzaldehyde produces benzyl alcohol and sodium benzoate in the presence of sodium hydroxide.
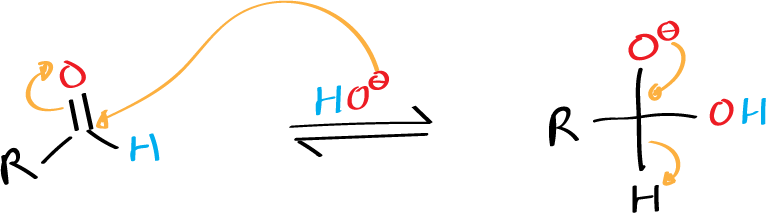
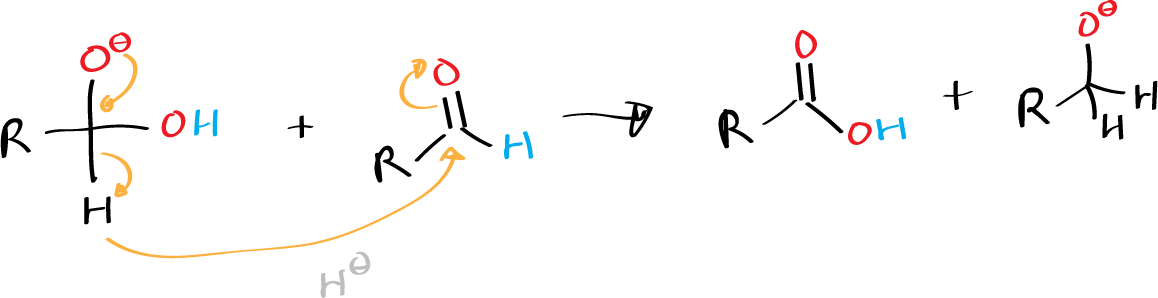
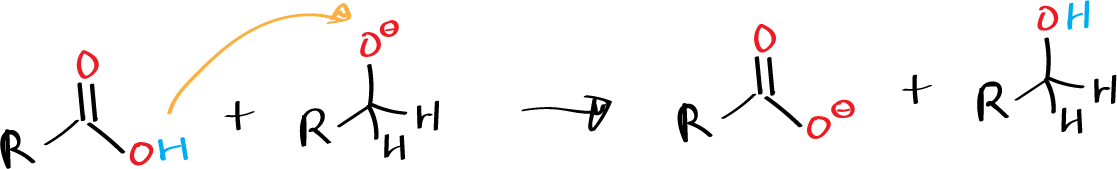
Formaldehyde also undergoes Cannizzaro reaction, as do aliphatic aldehydes (such as trimethylacetaldehyde) that do not contain α-hydrogens, and aromatic derivatives such as benzaldehyde.
Experimental procedure
In an Erlenmeyer flask of 250 ml place 10 ml (10.4 g, 98 mmol) of benzaldehyde and 9 g of NaOH (dissolved in 5 ml of water). To cover with a stopper (septum) firmly, and to shake, during 20 min, vigorously with the hand, until a solid product and a colorless liquid appear simultaneously.
Once concluded the reaction, sufficient water is added until dissolving the solid that has been formed (sodium benzoate), but avoiding an excess of water. To take the solution to a funnel of decanting, to wash the flask with some milliliters of CH2Cl2, to drag the remains of solid that have remained in the flask and to extract the solution with CH2Cl2 (3 x 15 ml).
To keep the phases, and to reunite the organic extracts. Then wash with 10 % NaHCO3 (2 x 20 ml) and 10 % Na2CO3 (1 x 20 ml). Then dry over anhydrous Na2SO4, filter and evaporate the CH2Cl2 at the rotary evaporator. Optionally, a subsequent vacuum distillation can be carried out to purify the obtained benzyl alcohol (colorless liquid slightly soluble in water).
The alkaline aqueous layer of the first extraction is acidified with HCl, precipitating the benzoic acid as a white mass. The mixture is cooled, filtered under vacuum and washed with water. Benzoic acid is a white, crystalline solid, slightly soluble in water (0.2 g/100 g at 20 °C, 2.2 g/100 g at 75 °C). It can be recrystallized in water to obtain pure benzoic acid (estimated yield of 80%).
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Benzaldehyde | 106.12 | -26 | 178-179 | 1.044 |
| Benzoic acid | 122.12 | 125 | 249 | 1.08 |
| Benzyl alcohol | 108.140 | -15 | 203-205 | 1.040 |
| CH2Cl2 | 84.93 | -97 | 40.0 | 1.33 |
| HCl | 36.46 | -30 | >100 | 1.200 |
| Na2CO3 | 105.99 | 851 | - | 2.532 |
| Na2SO4 | 142.04 | 884 | - | 2.630 |
| NaHCO3 | 84.01 | 300 | - | 2.160 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
| Sodium benzoate | 144.1 | >300 | - | 1.440 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Benzaldehyde |  |
| Benzoic acid |   |
| Benzyl alcohol |  |
| CH2Cl2 |  |
| HCl |   |
| Na2CO3 |  |
| Na2SO4 | Non-hazardous |
| NaHCO3 | Non-hazardous |
| NaOH |  |
| Sodium benzoate |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Benzaldehyde | HUMNYLRZRPPJDN-UHFFFAOYSA-N |
| Benzoic acid | WPYMKLBDIGXBTP-UHFFFAOYSA-N |
| Benzyl alcohol | WVDDGKGOMKODPV-UHFFFAOYSA-N |
| CH2Cl2 | YMWUJEATGCHHMB-UHFFFAOYSA-N |
| HCl | VEXZGXHMUGYJMC-UHFFFAOYSA-N |
| Na2CO3 | CDBYLPFSWZWCQE-UHFFFAOYSA-L |
| Na2SO4 | PMZURENOXWZQFD-UHFFFAOYSA-L |
| NaHCO3 | UIIMBOGNXHQVGW-UHFFFAOYSA-M |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
| Sodium benzoate | WXMKPNITSTVMEF-UHFFFAOYSA-M |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Vogel, A.I., Furniss, B.S., Hannaford, A.J., Tatchell, A.R., and Smith, P.W.G. (1989). Vogel’s Textbook of Practical Organic Chemistry (Vogel’s Textbook series). Longman. ISBN: 9780470214145