What is acetoacetic ester synthesis?
The acetoacetic ester synthesis is a chemical process that involves the base-catalyzed alkylation or arylation of β-ketoesters, which are then subjected to mild hydrolysis and decarboxylation to yield substituted acetones. Alternatively, treatment with concentrated base can produce substituted esters. Despite being first reported by Simonsen in 1908, the reaction was not officially named.
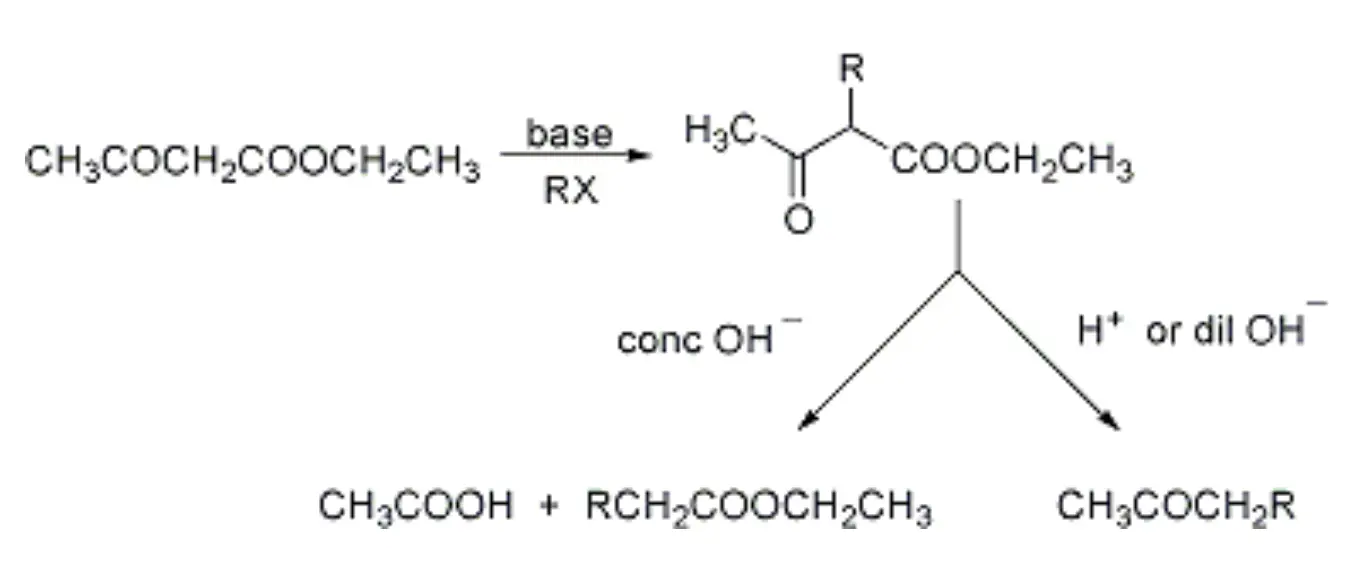
Due to their proximity to two electron-withdrawing groups (namely, the carbonyl and ester groups), the α-methylene protons found in β-ketoesters (such as acetoacetic esters) are highly acidic, with a pKa value that can be as low as 10. As a result, the α-methylene proton is easily deprotonated, leading to the formation of an α-carbanion that can be alkylated or acylated.
Alternatively, acetoacetic esters can also be alkylated under acidic conditions, via the intermediate enol form. However, this may result in the formation of unexpected products. The resulting β-substituted β-ketoesters can then be treated with a concentrated base (typically a strong base) to yield substituted esters, or hydrolyzed under mild (acidic or basic) conditions to produce substituted acetones via decarboxylation.
References
Simonsen, J.L.,”CLXXV.—Syntheses with the aid of monochloromethyl ether. Part I. The action of monochloromethyl ether on the sodium derivatives of ethyl malonate and ethyl isopropylmalonate” J. Chem. Soc., Trans., 1908, 93, 1777-1789
DOI: 10.1039/CT9089301777