What is acyloin condensation?
Acyloin condensation is the reductive coupling of esters by sodium to yield acyloins (α-hydroxyketones). Yields are greatly improved in the presence of trimethylchlorosilane.
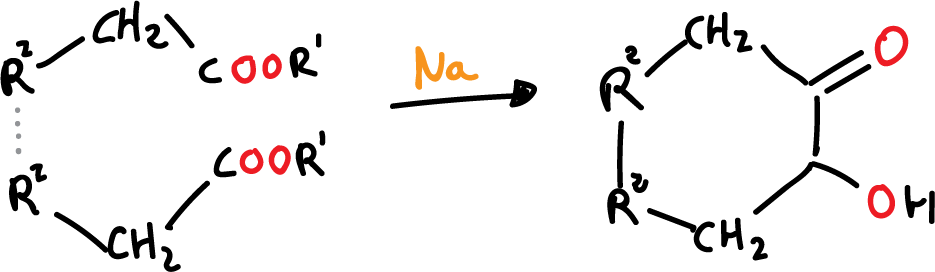
Mechanism of reaction
The mechanism of the acyloin condensation can be described as follows:
- Deprotonation of the carboxylic acid: The first step in the acyloin condensation is the deprotonation of the carboxylic acid by a strong base, such as sodium hydroxide or potassium hydroxide. This step generates a carboxylate anion, which is an intermediate compound in the reaction..
- Formation of the beta-diketone intermediate: The carboxylate anion then reacts with an aldehyde or ketone to form a beta-diketone intermediate compound. This intermediate compound has a carbon-carbon double bond adjacent to a ketone or aldehyde group, and is known as an acyloin..
- Ring closure: The beta-diketone intermediate undergoes a ring closure reaction, in which the carbon-carbon double bond is reduced and the two carbonyl groups become bonded to the same carbon atom. This results in the formation of the acyloin compound..
- Formation of water: The acyloin condensation reaction generates a molecule of water as a byproduct. This water molecule is formed from the deprotonation of the carboxylic acid in the first step of the reaction..
Overall, the acyloin condensation involves the deprotonation of a carboxylic acid, the formation of a β-diketone intermediate compound, and the ring closure of the intermediate to form the acyloin. The reaction is typically carried out in the presence of a strong base and a solvent, and can also be catalyzed by Lewis acids or Bronsted acids to improve the yield and selectivity of the reaction..
References
L. Bouveault, R. Loquin, Compt. Rend. 140, 1593 (1905)