What is alkyne zipper reaction?
The alkyne zipper reaction, also known as the Brown alkyne zipper reaction or alkyne isomerization reaction or alkyne-allene rearrangement, is a chemical process that involves the isomerization of internal alkynes into terminal alkynes by treating them with potasium 3-aminopropylamide (KAPA) or lithium 3-aminopropylamide (LiAPA) (see list of acronyms).
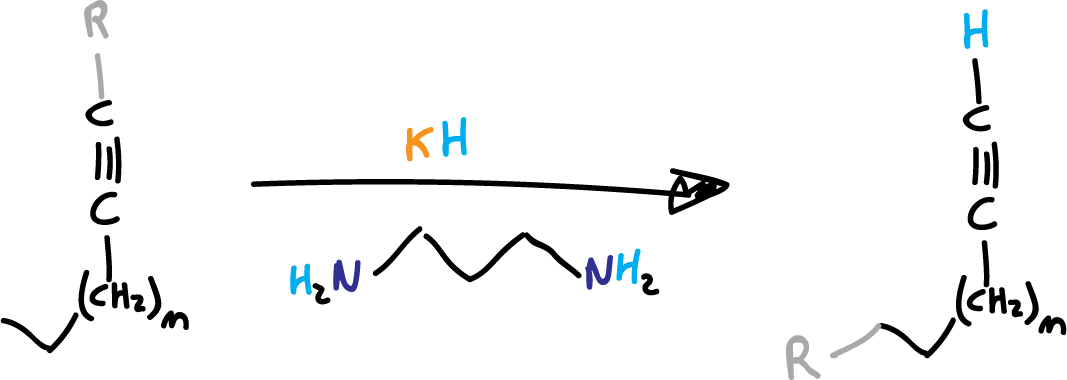
The alkyne zipper reaction was initially mentioned by A. Favorsky (Favorskii) in 1888. However, the specific variation presented here was likely first described in 1975.
References
- Faworsky, A. (1888), Isomerisationserscheinungen der Kohlen-wasserstoffe CnH2n−2. [Isomerization phenomena of the carbon-hydrogens CnH2n−2] J. Prakt. Chem., 37: 382-395. https://doi.org/10.1002/prac.18880370133
- Faworsky, A. (1888), Isomerisationserscheinungen der Kohlen-wasserstoffe CnH2n−2. (Zweite Abhandlung). [Isomerization phenomena of the carbon-hydrogens CnH2n−2] J. Prakt. Chem., 37: 417-431. https://doi.org/10.1002/prac.18880370138
- Faworsky, A. (1888), Isomerisationserscheinungen der Kohlen-wasserstoffe CnH2n−2. Dritte Abhandlung. [Isomerization phenomena of the carbon-hydrogens CnH2n−2] J. Prakt. Chem., 37: 531-536. https://doi.org/10.1002/prac.18880370147
- Saline hydrides and superbases in organic reactions. IX. Acetylene zipper. Exceptionally facile contrathermodynamic multipositional isomeriazation of alkynes with potassium 3-aminopropylamide
Charles Allan Brown and Ayako Yamashita
Journal of the American Chemical Society 1975 97 (4), 891-892
DOI: 10.1021/ja00837a034
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.