Written by J.A Dobado | Last Updated on May 2, 2024
What is Bakelite process?
Phenol alcohol, also known as orthohydroxy benzyl alcohol, oxybenzylalcohol, or saligenin, was first isolated by Piria in 1843. When heated, it can be converted into a resin known as “saliretins.”
The reaction between phenol and aldehyde was first reported by von Baeyer (Nobel Prize in Chemistry 1905) in 1872, who used an iron pressure cooker he invented, called a “bakelizer,” which resulted in a sticky substance he dismissively called “Schmiere.”
The actual reaction between formaldehyde and phenol was independently studied by Manasse and Lederer in 1894, after formaldehyde became a commercial product. In Manasse’s method, phenol was treated with an equivalent of strong alkali base in aqueous solution for several days until the odor of formaldehyde disappeared. In Lederer’s method, phenol was heated with weak base until the reaction was complete.
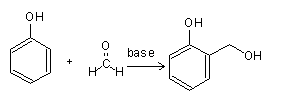
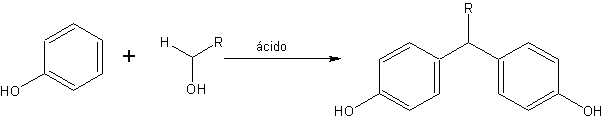
As a result, the reaction of phenol and formaldehyde under basic conditions is known as the Manasse-Lederer reaction. The resulting products, known as “shellac substitutes,” are soluble in alcohol, acetone, and alkaline hydroxide and melt on heating, resolidifying after cooling. This heating and melting can be repeated indefinitely.
Baekeland developed a process to make phenolic thermosetting resin from phenol and formaldehyde in 1909, using an apparatus that provided external pressure to balance the internal pressure. Formaldehyde was heated with an excess of phenol and an acid condensing agent to form fusible, soluble resins.
The process consists of three phases.
- The product from the first phase, called the initial condensation product, is designated Bakelite A. At ordinary temperatures, Bakelite A may be liquid, viscous, pasty, or solid and is soluble in alcohol, acetone, phenol, and NaOH solution.
- The product from the second phase, the intermediate condensation product, is designated Bakelite B. It is solid at all temperatures and is insoluble in all solvents but may swell in acetone, phenol, or terpineol. It softens during heating and becomes elastic but does not melt and becomes hard and brittle upon cooling.
- The product from the third phase is called the final condensation product, or Bakelite C. It is infusible, insoluble in all solvents, and indifferent to ordinary acids and alkaline solutions. Other properties of Bakelite C include poor conductivity of heat and electricity and excellent thermal stability.
The process to make Bakelite is referred to as the Bakelite process, and Bakelite is the trademark of oxybenzylmethylen-glycolanhydride.
References
- Baeyer, A. (1872), Ueber die Verbindungen der Aldehyde mit den Phenolen. [On the compounds of aldehydes with phenols] Ber. Dtsch. Chem. Ges., 5: 280-282. https://doi.org/10.1002/cber.18720050186
- Baeyer, A. (1872), Ueber die Verbindungen der Aldehyde mit den Phenolen und aromatischen Kohlenwasserstoffen. [On the compounds of aldehydes with phenols and aromatic hydrocarbons.] Ber. Dtsch. Chem. Ges., 5: 1094-1100. https://doi.org/10.1002/cber.187200502157
- Manasse, O. (1894), Ueber eine Synthese aromatischer Oxyalkohole. [On a synthesis of aromatic oxyalcohols.] Ber. Dtsch. Chem. Ges., 27: 2409-2413. https://doi.org/10.1002/cber.189402702239
- Manasse, O., “Process of making phenol alcohol” U.S. Patent, (1894) US 526786
- Lederer, L. (1894), Eine neue Synthese von Phenolalkoholen. [A new synthesis of phenolic alcohols.] J. Prakt. Chem., 50: 223-226. https://doi.org/10.1002/prac.18940500119
- Lederer, L., U.S. Patent., “Process of obtaining oxybenzylic alcohol” (1896) US 563975
- Baekeland, L. H., J. Ind. Eng. Chem., 1909, 1, 149
- Baekeland, L. H., J. Ind. Eng. Chem., 1911, 3, 932