Written by J.A Dobado | Last Updated on May 2, 2024
What is Traube purine synthesis?
The Traube reaction, also known as the Traube purine synthesis, is a method for synthesizing purine derivatives from 4-amino-6-hydroxypyrimidine or 4,6-diaminopyrimidine. The reaction involves introducing the amino group into the 5-position of the pyrimidines through nitrosation and reduction with ammonium sulfide (NH4)2S. Ring closure is achieved using either formic acid HCOOH or chlorocarbonic ester.
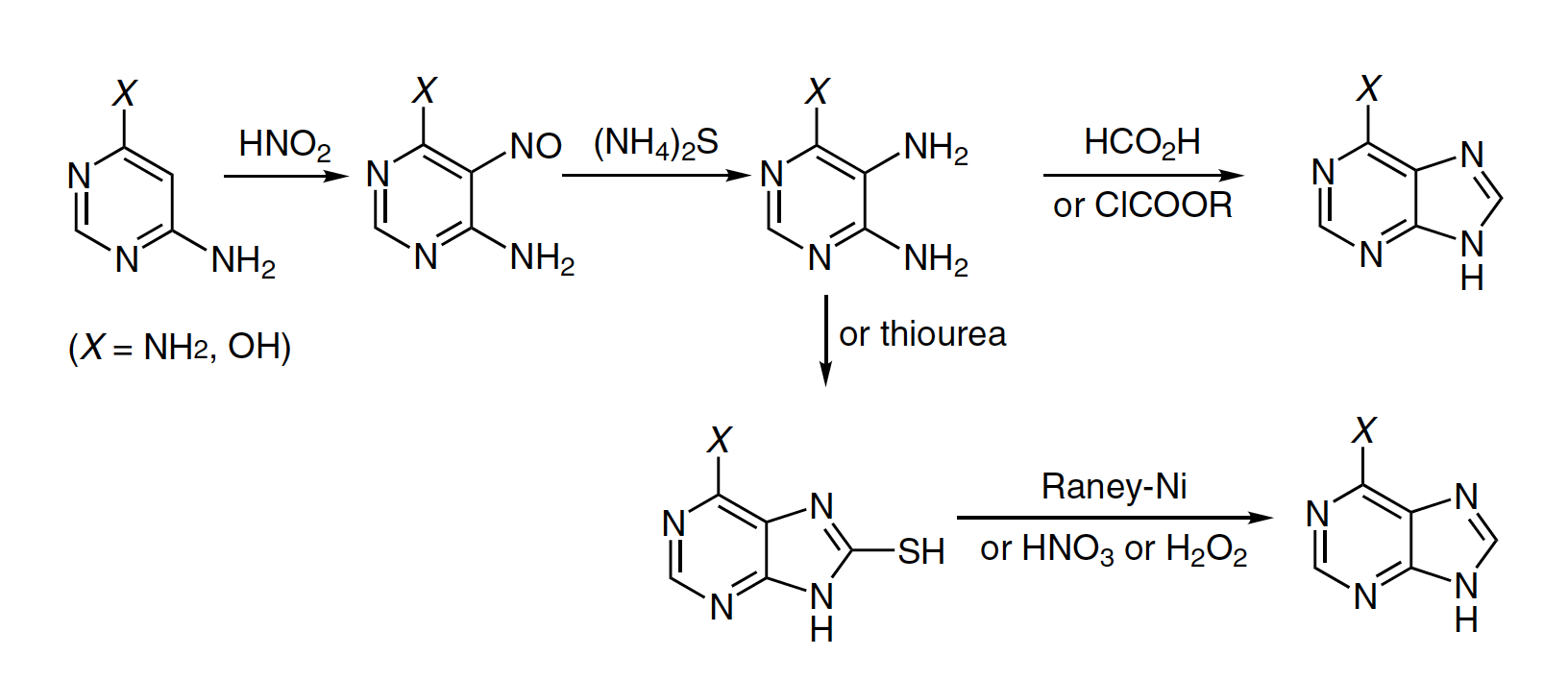
However, the use of impure starting materials can lead to unexpected product formation, and even with pure pyrimidines, the reaction may only proceed to the formylation stage. An alternative method involves cyclizing 4,5-diaminopyrimidines with urea or potassium ethyl xanthate to form 8-oxo and 8-thioxo derivatives, which can be further processed through desulfurization or oxidation.
Purines can also be synthesized through coupling with aldehydes in either a two-step process with ferric chloride or copper acetate or a one-step process via oxidative cyclization. It is important to avoid the 4-alkoxyamino group as it is reduced during the reaction, preventing the preparation of 9-hydroxypurine derivatives.
References
- Traube, W. (1900), Ueber eine neue Synthese des Guanins und Xanthins. [On a new synthesis of guanine and xanthine] Ber. Dtsch. Chem. Ges., 33: 1371-1383. https://doi.org/10.1002/cber.190003301236
- Traube, W. (1900), Der synthetische Aufbau der Harnsäure, des Xanthins, Theobromins, Theophyllins und Caffeïns aus der Cyanessigsäure. [The synthetic structure of uric acid, xanthine, theobromine, theophylline and caffeic acid from cyanoacetic acid] Ber. Dtsch. Chem. Ges., 33: 3035-3056. https://doi.org/10.1002/cber.19000330352
- Traube, W. (1904), Der Aufbau der Xanthinbasen aus der Cyanessigsäure. Synthese des Hypoxanthins und Adenins. [The structure of xanthine bases from cyanoacetic acid. Synthesis of hypoxanthine and adenine] Justus Liebigs Ann. Chem., 331: 64-88. https://doi.org/10.1002/jlac.19043310106