What are benzidine and semidine rearrangements?
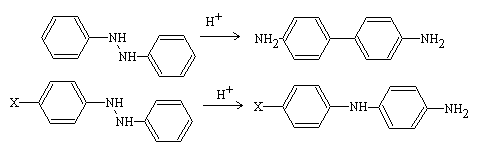
The acid-catalyzed rearrangement of hydrazobenzenes to 4,4′-diaminobiphenyls is referred to as the Benzidine rearrangement. Additionally, the semidine rearrangement occurs when a para substituent is present on the hydrazobenzene, resulting in the formation of p-aminodiphenylamine as the preferred product. These rearrangements are collectively known as the Zinin rearrangement, named after the Russian chemist Nikolai Nikolaevich Zinin (1812-1880).
References
- Zinin, N. , Über das Azobenzid und die Nitrobenzinsäure. [On the azobenzide and nitrobenzoic acid] J. Prakt. Chem., 36: 93-107 (1845). https://doi.org/10.1002/prac.18450360125
- A.W. Hofmann, “Notes of Researches on the Polyammonias, No. XXIII.–Hydrazobenzol, a New Compound Isomeric with Benzidine” Proc. Roy. Soc. London 12, 576-578 (1863)
- P. Jacobson, F. Henrich, J. Klein, “Untersuchungen über Reductionsproducte von Azoverbindungen. III” [Studies on reduction products of azo compounds. III] Ber. Dtsch Chem. Ges., 26, 688 (1893)
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.