Written by J.A Dobado | Last Updated on May 2, 2024
What is Ciamician-Dennstedt rearrangement?
The Ciamician-Dennstedt rearrangement is a chemical reaction that involves the transformation of a pyrrole ring (a five-membered ring) into a six-membered ring by heating it in an alkaline solution with chloroform, CHCl3, or other halogeno compounds. During the reaction, an intermediate product called dichlorocarbene CH2Cl2 is formed, which then reacts with the pyrrole to form an unstable dihalogenocyclopropane. The dihalogenocyclopropane rearranges further to produce a 3-halogenopyridine. It is important to note that the Ciamician-Dennstedt rearrangement is not catalyzed.
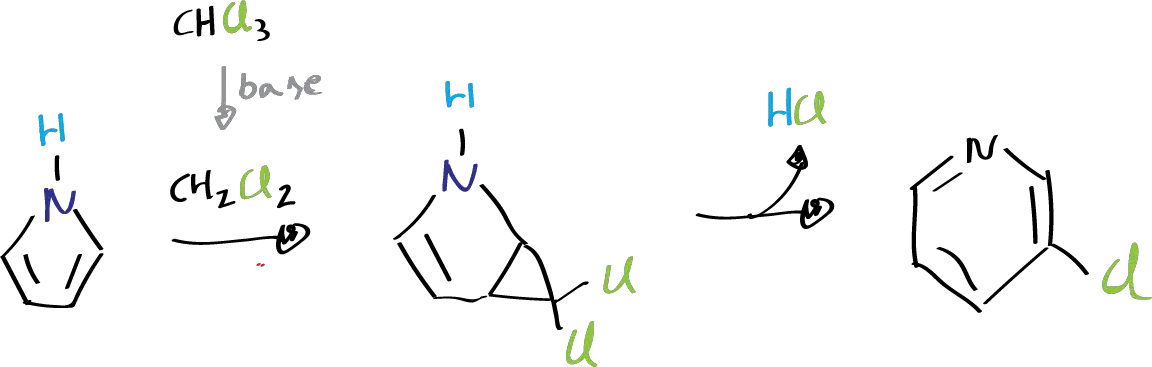
The Ciamician-Dennstedt rearrangement was first conducted by Ciamician and Dennstedt (Sapienza University of Rome) using the potassium salt of pyrrole and chloroform, CHCl3, in ether. After purification, they obtained the 3-chloropyridine compound, which was confirmed through crystallization with platinum. Although the pyrrole salt can be used as the base, it is more common to form the chloroform carbene with an alkali alcohol.
References
Ciamician, G.L. and Dennstedt, M. (1881), Ueber die Einwirkung des Chloroforms auf die Kaliumverbindung Pyrrols. [On the effect of chloroform on the potassium compound pyrrole.] Ber. Dtsch. Chem. Ges., 14: 1153-1163. https://doi.org/10.1002/cber.188101401240